Welcome to Mr Conroys Science Class Topic 1









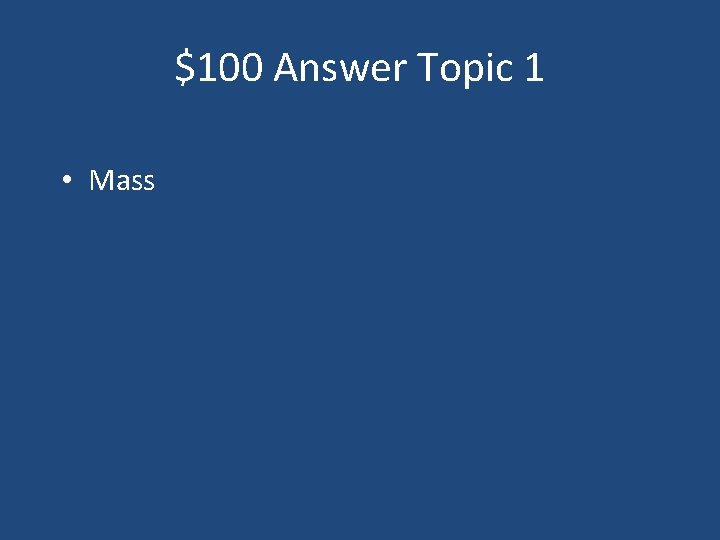


























































































- Slides: 104

Welcome to Mr. Conroy’s Science Class


Topic 1 What Matter Is

Topic 1 What Matter Is

Topic 2 What Matter is Made of

Topic 2 What Matter is Made of

Topic 3 Solids, Liquids, Gases

Topic 3 Solids, Liquids, Gases

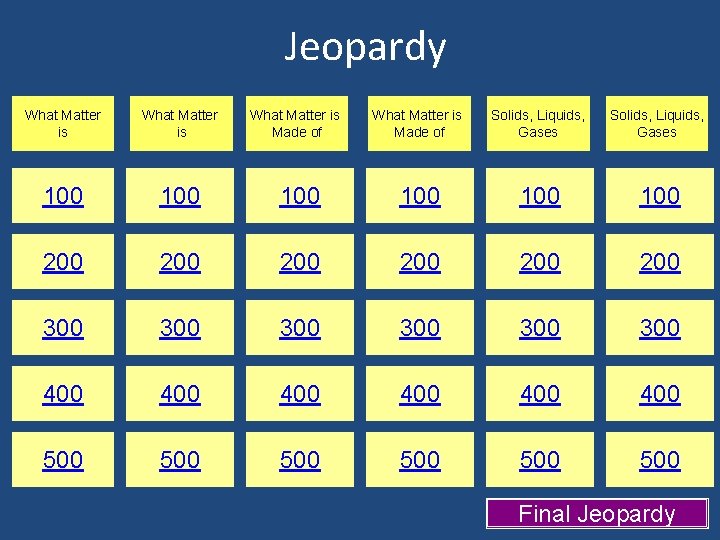
Jeopardy What Matter is 100 200 What Matter is Made of Solids, Liquids, Gases 100 100 200 200 200 300 300 300 400 400 400 500 500 500 Final Jeopardy

$100 Question Topic 1 1. The amount of matter in an object. a) Weight b) Mass c) Volume d) Density
$100 Answer Topic 1 • Mass

Return

$200 Question Topic 1 2. A graduated cylinder is used to measure_____. a) Weight b) Mass c) Volume d) Density

$200 Answer Topic 1 volume

Return

$300 Question Topic 1 5. A cube has a volume of 125 ml. It has a mass of 250 grams. The density of the cube is 50 g/ml and will float in water 2. 0 g/ml and will float in water 50 g/ml and will sink in water 2. 0 g/ml and will sink in water

$300 Answer Topic 1 2. 0 g/ml and will sink in water

Return

$400 Question Topic 1 The picture shows a cup containing three liquids and five different, round objects. The positions of these liquids and objects show their densities relate to one another. A less dense object or liquid will float on top of a denser object or liquid. C Can you determine that the two objects in the corn syrup, which are resting on the bottom of the cup, have the same densities? Explain your answ

$400 Answer Topic 1 No, you cannot tell if the two items have the same density because they are resting on the bottom. The only thing you can tell is that they are the most dense of all of the substances.

Return

$500 Question Topic 1 A student observes two blue spheres with the same circumference that look identical in size and shape. What can you conclude about the masses of spheres A and B? Explain your answer. What can you conclude about the volumes of the spheres? Explain your answer. (4 Points)

$500 Answer Topic 1 The masses of the two spheres are different because Is lower on the balance than the other. The volumes are the same because they are they Are the exact same size and shape.

Return

$100 Question Topic 2 The amount of space an object takes up. a)mass b)buoyancy c)volume d) weight

$100 Answer Topic 2 volume

Return

Daily Double

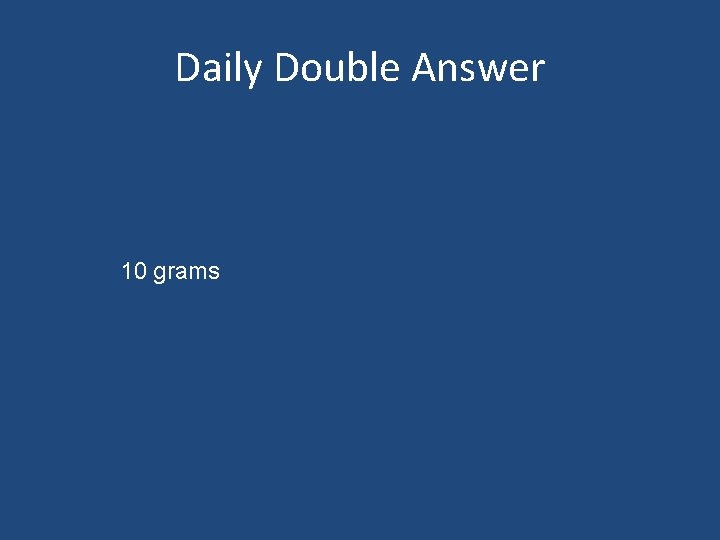
Daily Double Question A spring scale is used to measure the weight of an object on earth. The spring scale on earth reads 60 grams. On the moon the spring scale would read. a) 60 grams b) 10 grams c) 600 grams d) 0 grams

Daily Double Answer 10 grams

Return

$300 Question Topic 2 A measure of how tightly packed matter is; the amount of mass contained in a given volume. a)density b)mass c)volume d)weight

$300 Answer Topic 2 Density

Return

$400 Question Topic 2 9. A graduated cylinder contains 100 ml of a liquid. The mass of the graduated cylinder with the liquid is 145 grams. The mass of the graduated cylinder when empty is 45 grams. The liquid is most likely a) syrup-density of 24. 5 g/ml b) Water-density of 1. 0 g/ml c) motor Oil-density of. 75 g/ml d) salt water-density of 1. 45 g/ml

$400 Answer Topic 2 water-density of 1 g/ml

Return

$500 Question Topic 2 • What does it mean when materials conduct? Give two examples. • What does it mean when materials insulate? Give two examples.

$500 Answer Topic 2 Conduct- when heat and or electricity can flow through easily Insulate- when heat and or electricity cannot flow through as easy Examples will vary

Return

$100 Question Topic 3 What are elements?

$100 Answer Topic 3 elements are the purest substances found on earth

Return

$200 Question Topic 3 What is a compound? Give an example.

$200 Answer Topic 3 compounds are two or more different elements joined together to form a solid, liquid, or gas

Return

$300 Question Topic 3 Draw an atom of the element carbon (atomic number 6)

$300 Answer Topic 3 www. chemicalelements. com

Return

$400 Question Topic 3 Explain how you can determine the number of neutrons of an element

$400 Answer Topic 3 subtract the atomic number from the atomic mass

Return

$500 Question Topic 3 Number First Energy Level: 2 Second Energy Level: 8 Third Energy Level: 18 Fourth Energy Level: 1 What is the electron configuration of This element?

Isotopes $500 Answer Topic 3 2, 8, 1

Return

$100 Question Topic 4 Missi filled some soda bottles halfway with water and left them outside overnight. The temperature dropped below the freezing point. Which of following will describe the water in the morning? A. The water will change from a solid to a gas. B. The water will change from a gas to a liquid. C. The water will change from a liquid to a solid. D. The water will change from a liquid to a gas.

$100 Answer Topic 4 C. The water will change from a liquid to a solid

Return

$200 Question Topic 4 4. 5. Tim has two identical wooden blocks. He placed one of the wooden blocks into Liquid A, and the other wooden block into Liquid B. Liquid A Which is a correct observation? Liquid B A. The wooden block in Liquid A sank. B. The wooden block in Liquid B will float in Liquid A. C. The wooden block in Liquid A is heavier than the block in Liquid B. D. The wooden block in Liquid A floated.

$200 Answer Topic 4 A. The wooden block in Liquid A sank

Return

$300 Question Topic 4 3. Jen got a balloon after eating dinner at a restaurant. 3. While still inside the restaurant her balloon looked like the one shown to the right: A. Balloon 1 B. Balloon 2 C. Balloon 3 1 a 1. 22 2. 33 3. 44 4. D. Balloon 4 What will her balloon look like when she goes outside into the cold, winter night?

$300 Answer Topic 4 B-balloon 2

Return

$400 Question Topic 4 On a hot, sunny day, Lee is enjoying a glass of lemonade while sitting in a lawn chair. A picture is taken of the glass when she first goes outside. Another picture is taken 15 minutes later. Describe all the physical changes that have taken place. Describe the energy force or flow that caused the changes. (4 Points)

$400 Answer Topic 4 Physical changes are the ice melting and air outside turning from a gas to a liquid on the side of the glass (condensation) the air surrounding the glass is the cause for the changes

Return

$500 Question Topic 4 Explain how the difference between an open system and a closed system.

$500 Answer Topic 4 Open systems allow for matter escape and the mass change Closed systems contain matter and do not allow for things to escape

Return

$100 Question Topic 5 When a snowman melts, what change of matter can be seen taking place? ¡ A. Gas to liquid ¡ B. Liquid to gas ¡ C. Solid to liquid ¡ D. Liquid to solid

$100 Answer Topic 5 C. Solid to liquid

Return

$200 Question Topic 5 Val is pouring equal amounts of oil and vinegar into a glass. The vinegar sank to the bottom while the oil floated on top. Which of the following is the correct conclusion? A. In one hour they will mix together. B. The vinegar is denser than the oil. C. In one hour there will be less oil. D. The oil is denser than the vinegar.

$200 Answer Topic 5 B. The vinegar is denser than the oil.

Return

$300 Question Topic 5 Using what you know about the properties of heat, describe two things that could happen to a substance when it is heated. (2 Points)

$300 Answer Topic 5 expand, melt, molecules could speed up, bend, burn

Return

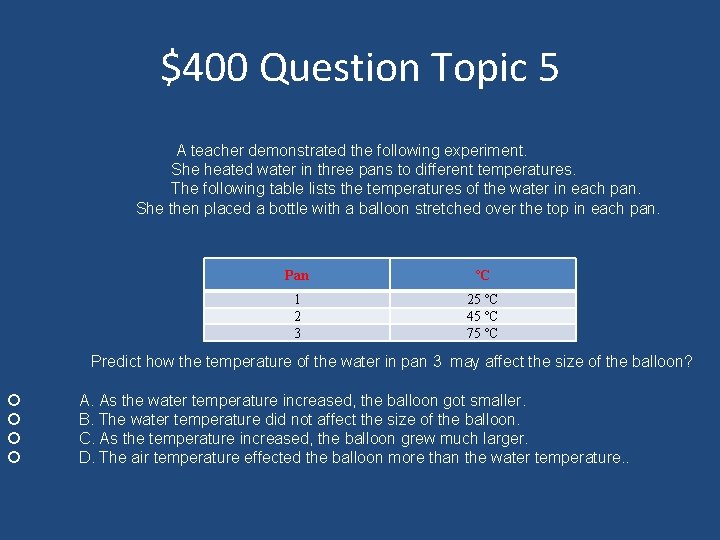
$400 Question Topic 5 A teacher demonstrated the following experiment. She heated water in three pans to different temperatures. The following table lists the temperatures of the water in each pan. She then placed a bottle with a balloon stretched over the top in each pan. Pan ºC 1 2 3 25 ºC 45 ºC 75 ºC Predict how the temperature of the water in pan 3 may affect the size of the balloon? A. As the water temperature increased, the balloon got smaller. B. The water temperature did not affect the size of the balloon. C. As the temperature increased, the balloon grew much larger. D. The air temperature effected the balloon more than the water temperature. .

$400 Answer Topic 5 C. As the temperature increased, the balloon grew much larger

Return

$500 Question Topic 5 Antonio blows up a balloon to 4000 cubic centimeters (cm 3). Every two hours he moves the balloon to rooms with different air temperatures. At the end of each two-hour session, Antonio measures the volume of his balloon in that particular room. Antonio’s results are recorded in the table below: What was the volume in Room 4? Explain what happens to gases as temperature decreases. A. 3025 cubic centimeters B. 3030 cubic centimeters C. 3020 cubic centimeters D. 3080 cubic centimeters Room Temperature Volume 1 2 3 4 5 40ºC 30ºC 20ºC 10ºC 4000 cm 3 3075 cm 3 3050 cm 3 ? cm 3 3000 cm 3

$500 Answer Topic 5 A. 3025 cubic centimeters As temperature decreases, the particles in gases move slower and contract (move closer together)

Return

$100 Question Topic 6 How can you change a substance from one state to another state of matter?

$100 Answer Topic 6 add or remove heat

Return

$200 Question Topic 6 • • What happens to the molecules when heat is added? What happens to the molecules when heat is taken away?

$200 Answer Topic 6 heat added-molecule speed up and expand heat taken away-Molecule slow down and contract

Return

$300 Question Topic 6 • • What is meant by melting point? What is meant by boiling point? What is meant by freezing point?

$300 Answer Topic 6 melting point –temp. at which solid turns to a liquid boiling point- temp. at which a liquid changes to a gas freezing point- temp. at which a liquid changes to a solid

Return

$400 Question Topic 6 Which would contain more thermal energy, a rock heated near a camp fire for a long period of time with a smaller mass or a rock with a larger mass? . Explain.

$400 Answer Topic 6 The rock with the larger mass. The reason is the larger Rock has more mass to store thermal energy.

Return

$500 Question Topic 6 What is the difference between temperature and thermal energy?

$500 Answer Topic 6 Temperature is the average kinetic energy of a substance and can be measured Thermal energy is related to the temperature and mass of the substance

Return

Final Jeopardy Topic

Final Jeopardy Question Explain how the raisins move about in the Experiment with the ginger ale.

Final Jeopardy Answer • Answers will vary

Thank You for Playing