Welcome to AP Chemistry Scientific Method A method













- Slides: 24

Welcome to AP Chemistry

Scientific Method A method of solving problems/answering questions l Observation- what is seen or measured l Hypothesis- educated guess of why things behave the way they do. (possible explanation) l Experiment- designed to test hypothesis l leads to new observations, l and the cycle goes on l

Scientific Method l Theory (Model) – A set of tested hypotheses that give an overall explanation of some natural phenomenon why things behave the way they do – explains why something happens l Law – The same observation applied to many different systems – Example - Law of Conservation of Mass Laws are summaries of observations l

Scientific Method Theories have predictive value. l The true test of a theory is if it can predict new behaviors. l If the prediction is wrong, theory must be changed. l Theory- why l Law - how l

Significant Figures Meaningful digits in a MEASUREMENT l Exact numbers are have unlimited significant figures l If it is measured or estimated, it has sig. figs. l All numbers except zero are significant. l Some zeros are, some aren’t l

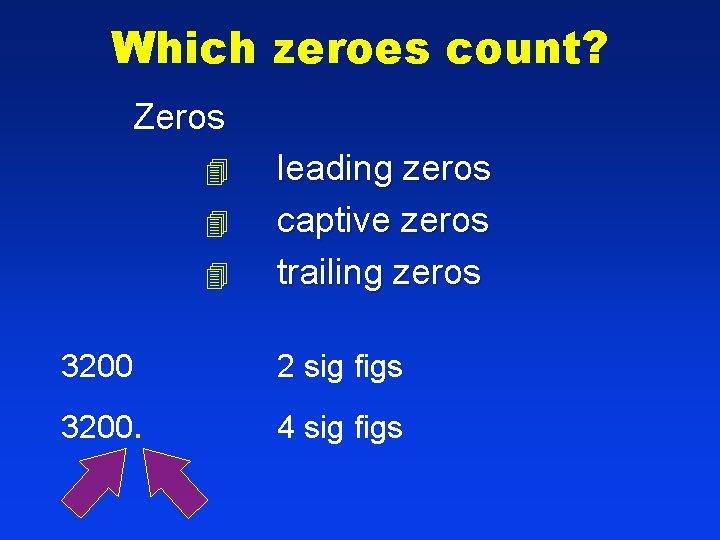
Which zeroes count? Zeros leading zeros captive zeros trailing zeros 3200 2 sig figs 3200. 4 sig figs

Which zeroes count? Zeros Captive zeros always count as significant figures. 16. 07 has 4 sig figs.

Which zeroes count? Zeros Trailing zeros are significant only if the number contains a decimal point. 9. 300 has 4 sig figs.

Which zeroes count? Exact numbers have an infinite number of significant figures. 1 inch = 2. 54 cm, exactly

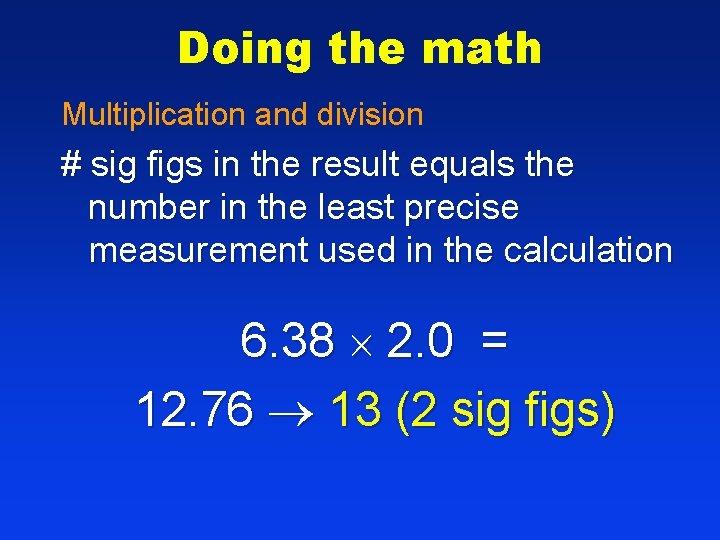
Doing the math Multiplication and division # sig figs in the result equals the number in the least precise measurement used in the calculation 6. 38 2. 0 = 12. 76 13 (2 sig figs)

Doing the math Addition and subtraction # sig figs in the result equals the number of decimal places in the least precise measurement. 6. 8 + 11. 934 = 22. 4896 22. 5 (3 sig figs)

SI Measurement System Every measurement has two parts – Number – Scale (unit) l SI system (le Systeme International) based on the metric system – Prefix + base unit – Prefix tells you the power of 10 to multiply by - decimal system -easy conversions l

The Fundamental SI Units

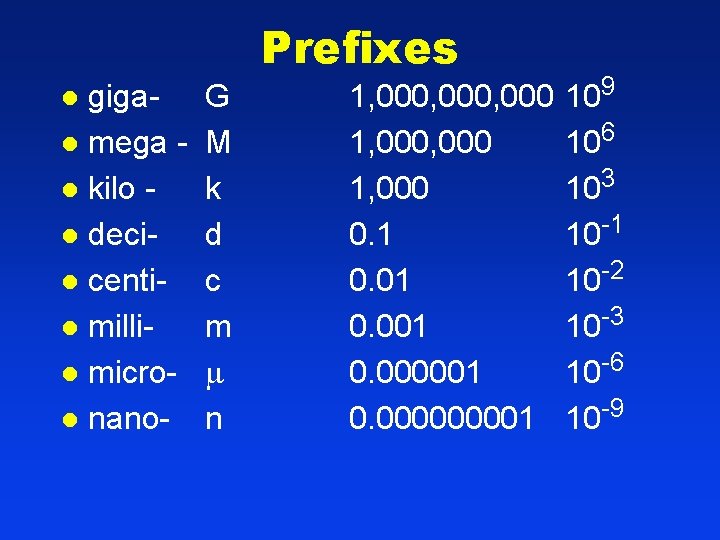
Prefixes gigal mega l kilo l decil centil millil microl nanol G M k d c m n 1, 000, 000 1, 000 0. 1 0. 001 0. 000000001 109 106 103 10 -1 10 -2 10 -3 10 -6 10 -9

Mass and Weight Mass is measure of resistance to change in motion l Weight is force of gravity. l Sometimes used interchangeably l Mass can’t change, weight can l

Uncertainty A digit that must be estimated is called uncertain. l A measurement always has some degree of uncertainty l Basis for significant figures l Precision- how repeatable l Accuracy- how correct - closeness to true value. l

Uncertainty l l Random error - equal chance of being high or low- addressed by averaging measurements – expected in all measurements Systematic error- always the same direction each time – you want to avoid this type of error you can have precision without accuracy You can’t have accuracy without precision

Dimensional Analysis Using the units to solve problems

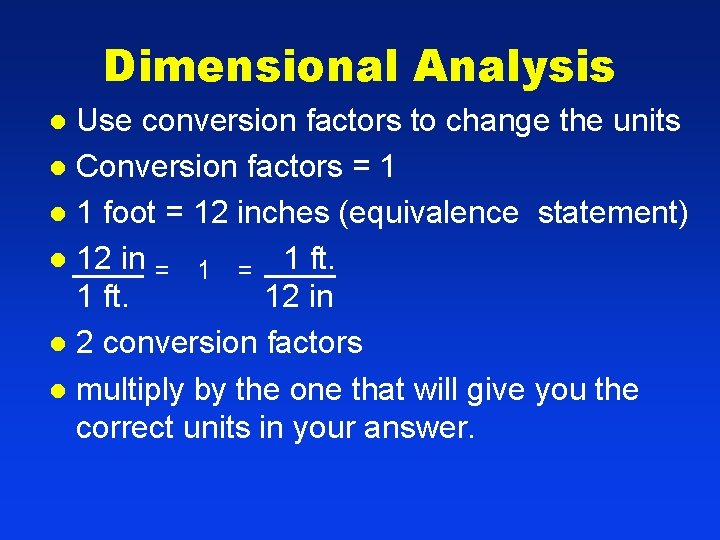
Dimensional Analysis Use conversion factors to change the units l Conversion factors = 1 l 1 foot = 12 inches (equivalence statement) l 12 in = 1 ft. 12 in l 2 conversion factors l multiply by the one that will give you the correct units in your answer. l

Examples Science fiction often uses nautical analogies to describe space travel. If the starship U. S. S. Enterprise is traveling at warp factor 1. 71, what is its speed in knots? l Warp 1. 00 = 5. 00 times the speed of light l speed of light = 3. 00 x 108 m/s l 1 knot = 2000 yd/h exactly l

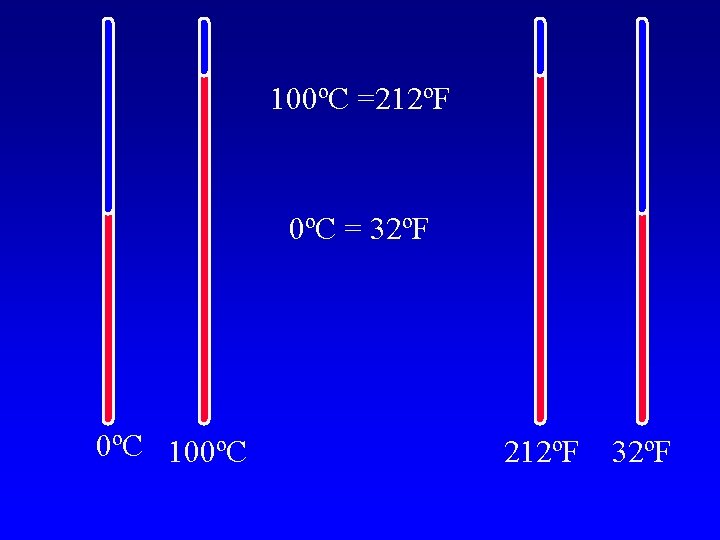
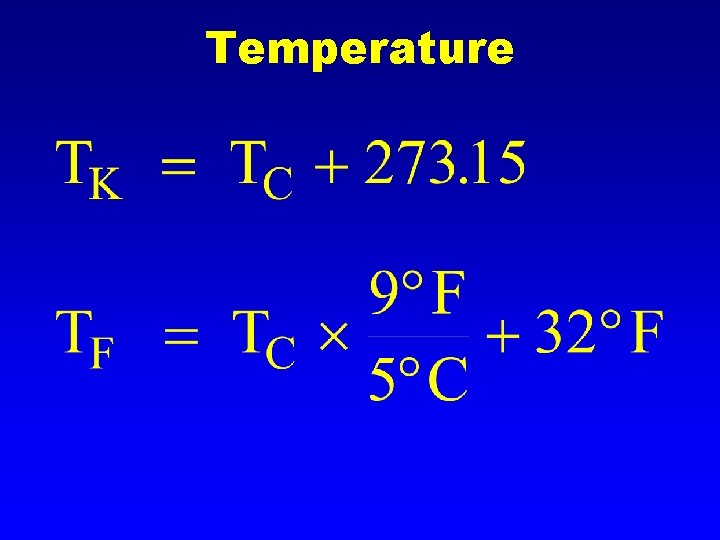
Temperature A measure of the average kinetic energy l Different temperature scales, all are talking about the same height of mercury. l

100ºC =212ºF 0ºC = 32ºF 0ºC 100ºC 212ºF 32ºF

Temperature

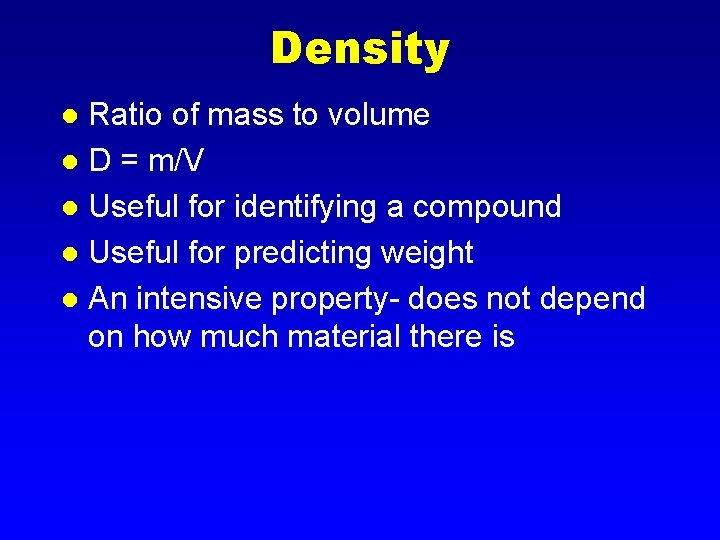
Density Ratio of mass to volume l D = m/V l Useful for identifying a compound l Useful for predicting weight l An intensive property- does not depend on how much material there is l