Welcome to ACST2 Guidance Useful Information ACST2 Office
















- Slides: 25

Welcome to ACST-2! Guidance & Useful Information ACST-2 Office Level 6, Nuffield Department of Surgical Sciences University of Oxford, John Radcliffe Hospital Headley Way, Oxford OX 3 9 DU, UNITED KINGDOM

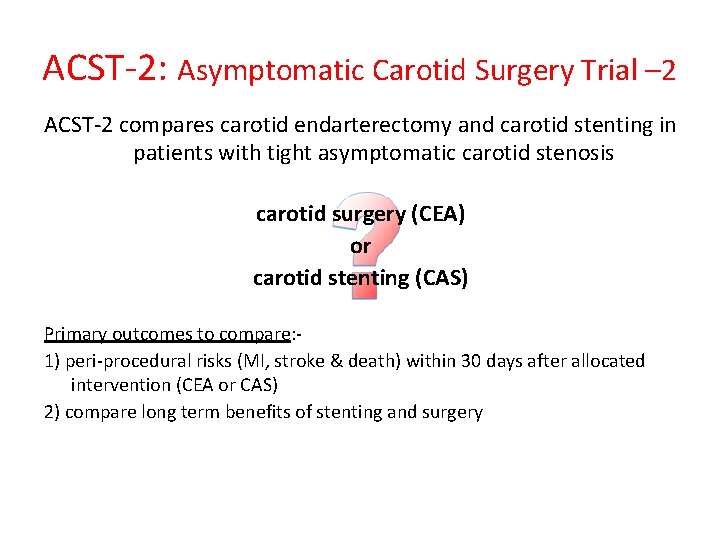
ACST-2: Asymptomatic Carotid Surgery Trial – 2 ACST-2 compares carotid endarterectomy and carotid stenting in patients with tight asymptomatic carotid stenosis carotid surgery (CEA) or carotid stenting (CAS) Primary outcomes to compare: - 1) peri-procedural risks (MI, stroke & death) within 30 days after allocated intervention (CEA or CAS) 2) compare long term benefits of stenting and surgery

ACST-2 Background First patient randomised: 15/01/2008 Patients are now in their 6 th year of follow up 92 active centres from 27 countries Largest (investigator-led) trial in the world comparing surgery vs. stenting in asymptomatic patients, having recruited >1, 520 • Goal: recruit over 3, 600 patients by the end of 2019 • Currently the peri-procedural stroke (fatal and disabling) and death within 30 days is 1. 0 % in 1078 patients • •

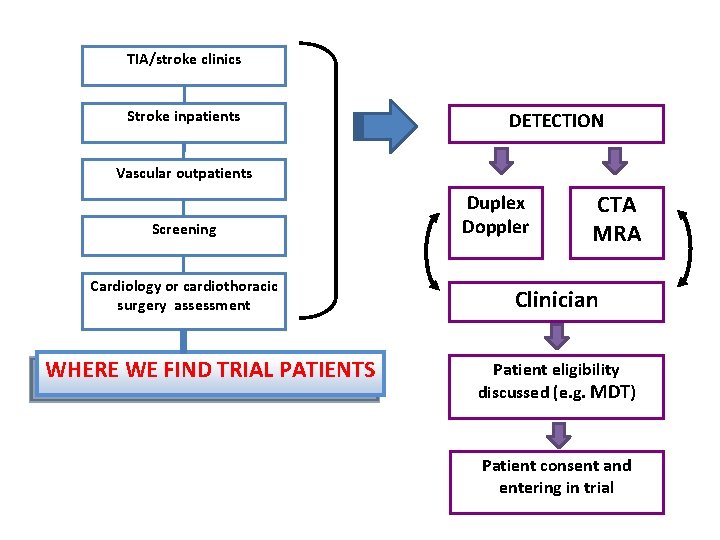
TIA/stroke clinics Stroke inpatients DETECTION Vascular outpatients Screening Cardiology or cardiothoracic surgery assessment WHERE WE FIND TRIAL PATIENTS Duplex Doppler CTA MRA Clinician Patient eligibility discussed (e. g. MDT) Patient consent and entering in trial

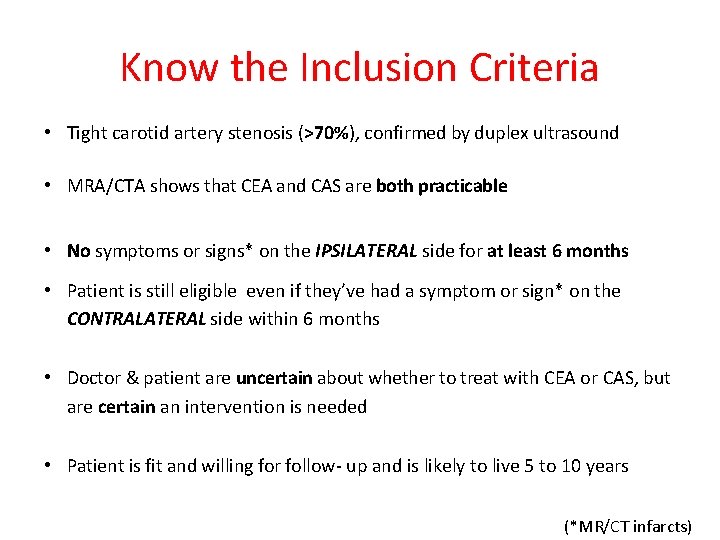
Know the Inclusion Criteria • Tight carotid artery stenosis (>70%), confirmed by duplex ultrasound • MRA/CTA shows that CEA and CAS are both practicable • No symptoms or signs* on the IPSILATERAL side for at least 6 months • Patient is still eligible even if they’ve had a symptom or sign* on the CONTRALATERAL side within 6 months • Doctor & patient are uncertain about whether to treat with CEA or CAS, but are certain an intervention is needed • Patient is fit and willing for follow- up and is likely to live 5 to 10 years (*MR/CT infarcts)

How to Randomise a patient in ACST-2 after informed consent 1. Web randomisation 2. Phone randomisation

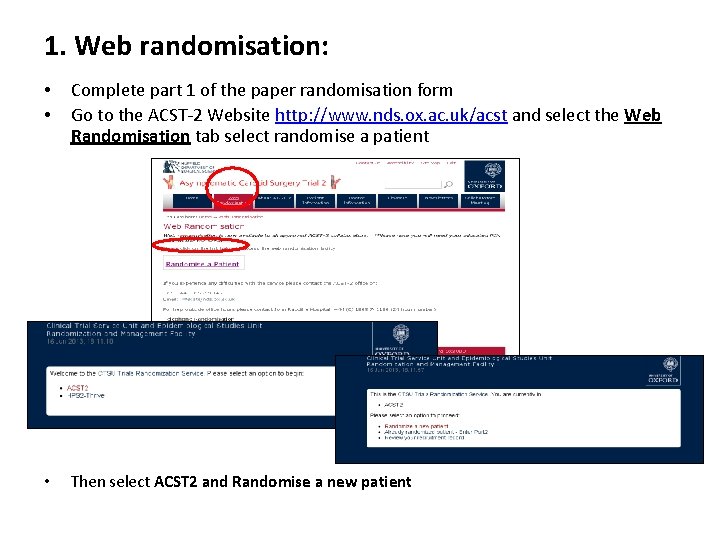
1. Web randomisation: • • Complete part 1 of the paper randomisation form Go to the ACST-2 Website http: //www. nds. ox. ac. uk/acst and select the Web Randomisation tab select randomise a patient • Then select ACST 2 and Randomise a new patient

Web randomisation cont. Ø Select your country and centre from the drop down list Ø Select your name and enter your unique PIN (XXXXXX) from the drop down list and press ‘continue’ Ø Select the name of the Randomising Doctor (if this isn’t you) Ø Then follow the instructions for on-line randomisation Ø Don’t forget to return a copy of the fully completed paper randomisation form to the ACST-2 Office!

2. Phone randomisation: Ø Complete part 1 of the randomisation form Ø Call +44 (0) 1865 765 615 (24 hour randomisation number) Ø Give the randomisation team your PIN number and confirm your Hospital details Ø The randomisation team will then guide you through telephone randomisation. Please remember: If you have any difficulties please call the ACST-2 office: +44 1865 221345 or email: acst@nds. ox. ac. uk Or outside of office hours: +44 (0) 1865 741166

Trial Documentation 1. Site file 2. Patient files 3. ACST-2 study forms (CRF)

1. Site file Contents • Contains all appropriate documentation according to GCP 1. Protocol/ protocol amendments (deviations/violations) 2. CRF 3. Patient information and consent forms 4. Investigator Section (agreements, finance, track records and MOI) 5. Ethics and R&D (submission, approval & annual reports) 6. Screening logs 7. Delegation log and CV of PI 8. Monitoring and Training 9. Correspondence 10. Note to file

2. Patient files • Set up patient files according to patient ID • Original CRF (consent, randomisation & 1 Month Follow-up form) to be stored in patient file • Copy sent to ACST-2 office • Any correspondence with the patient • Major events

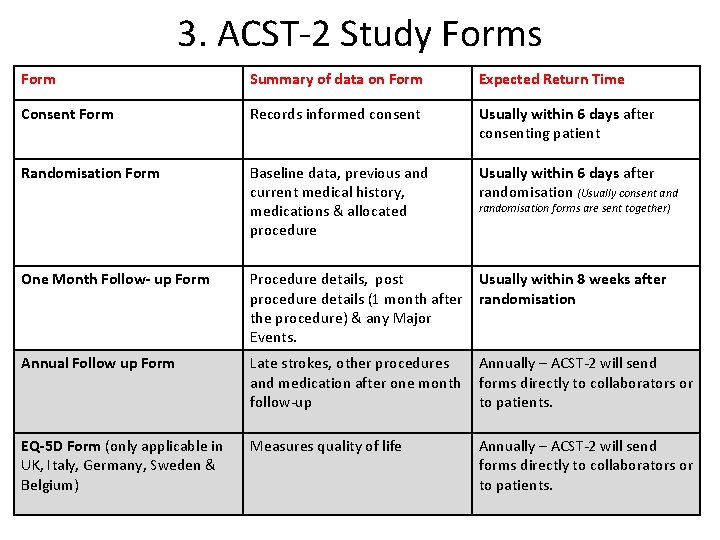
3. ACST-2 Study Forms Form Summary of data on Form Expected Return Time Consent Form Records informed consent Usually within 6 days after consenting patient Randomisation Form Baseline data, previous and current medical history, medications & allocated procedure Usually within 6 days after randomisation (Usually consent and randomisation forms are sent together) One Month Follow- up Form Procedure details, post Usually within 8 weeks after procedure details (1 month after randomisation the procedure) & any Major Events. Annual Follow up Form Late strokes, other procedures Annually – ACST-2 will send and medication after one month forms directly to collaborators or follow-up to patients. EQ-5 D Form (only applicable in UK, Italy, Germany, Sweden & Belgium) Measures quality of life Annually – ACST-2 will send forms directly to collaborators or to patients.

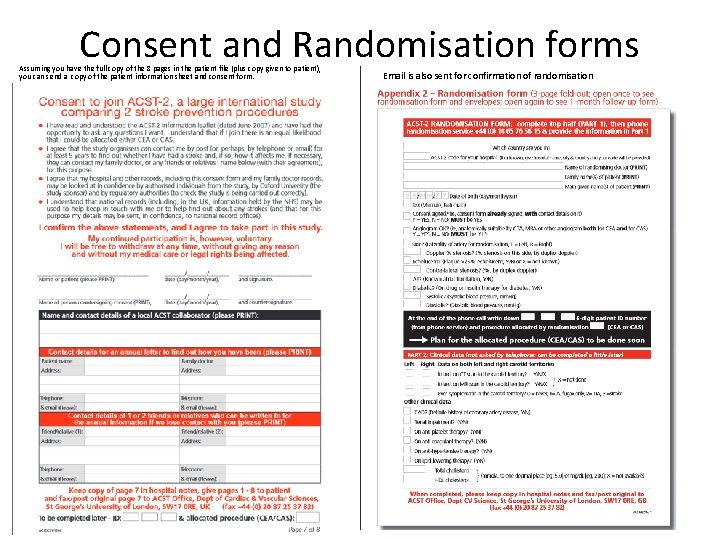
Consent and Randomisation forms Assuming you have the full copy of the 8 pages in the patient file (plus copy given to patient), you can send a copy of the patient information sheet and consent form. Email is also sent for confirmation of randomisation

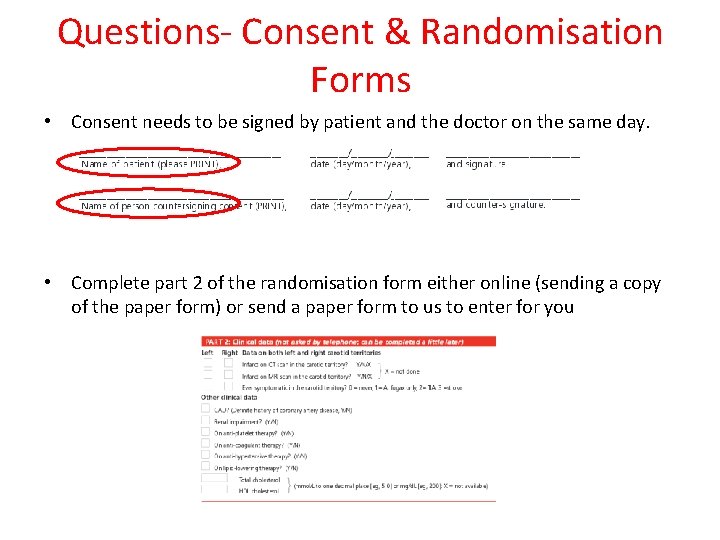
Questions- Consent & Randomisation Forms • Consent needs to be signed by patient and the doctor on the same day. • Complete part 2 of the randomisation form either online (sending a copy of the paper form) or send a paper form to us to enter for you

One Month form

Questions One month follow up form Section B The duplex Doppler must be done Left and Right sides. Section E Don’t forget to complete the date for Current Status. Current therapy please be sure to select Yes or No to each box – do not use crosses or ticks or leave blank. Only complete page two of the One Month Follow-up Form if there is a major event.

Common Queries • The patient cannot have the procedure due to a medical reason. Please record why on the one month follow up form on Page 2 under ‘Any additional comments or information (as narrative)? If the patient does end up having the procedure later at anytime (even years later), please complete a new One month Follow up Form with all the procedural details and send this to the ACST-2 office.

Common Queries • The patient withdraws from the procedure but not from the trial Please record this on the one month follow up form on Page 2 under ‘Any additional comments or information (as narrative)? Valuable information is provided even though the patient did not have the procedure.

Common Queries • A patient has a crossover due to a medical reason Please record why on the one month follow up form to note this on Page 2 under ‘Any additional comments or information (as narrative)? Then complete the rest of the form as per normal.

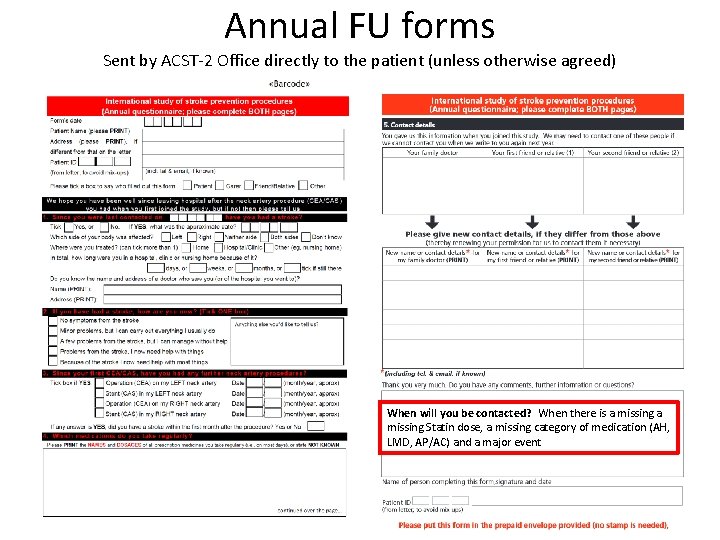
Annual FU forms Sent by ACST-2 Office directly to the patient (unless otherwise agreed) When will you be contacted? When there is a missing Statin dose, a missing category of medication (AH, LMD, AP/AC) and a major event

Common Queries- AF forms (rare) • Major Event • Normally we will contact the GP first or the patient again, but if needed we may ask you: 1)Statins –dosage 2) Why the patient is not on anti-hypertensive, lipid lowering medication or anti-platelet/anticoagulant

What are Major Events • • • Stroke MI Death Peri-procedural events (<30 days) or late events (>30 days). The information required about these events that you will need to send: • Stroke: • • *MI: Deaths: CT/MRI scan , initial and 6 month modified Rankin scores, clinical notes (duration of symptoms and treatment) ECGs, Cardiac markers, clinical notes Death certificate if available, cause of death, date of death Please report such events to acst@nds. ox. ac. uk as soon as possible (ideally within 1 week) * Only events within 30 days

Missing forms • All missing forms are reported to the Data Monitoring Committee that meet Annually

We are here to help! If you have any questions please contact us by email acst@nds. ox. ac. uk or call +44 (0) 1865 221 345