WELCO ME Karyotyping Chromosome Banding and Chromosome Painting

WELCO ME
Karyotyping, Chromosome Banding and Chromosome Painting
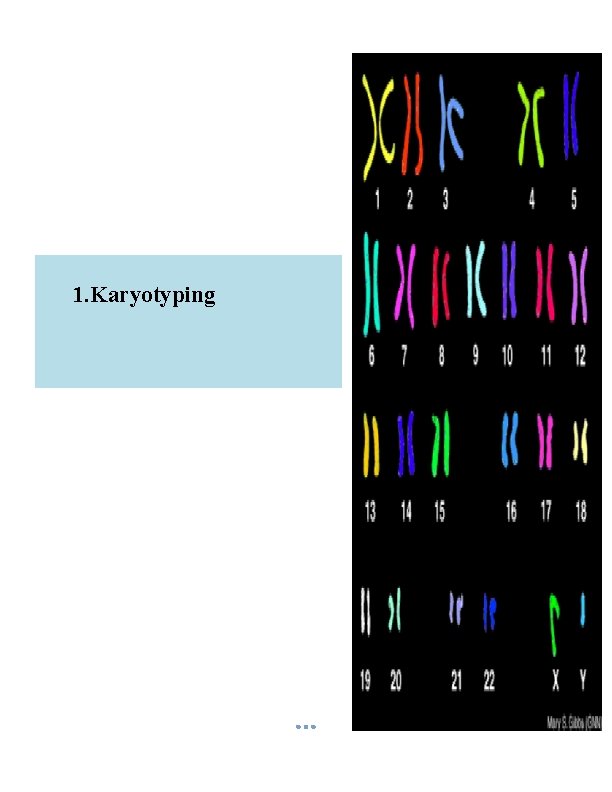
1. Karyotyping
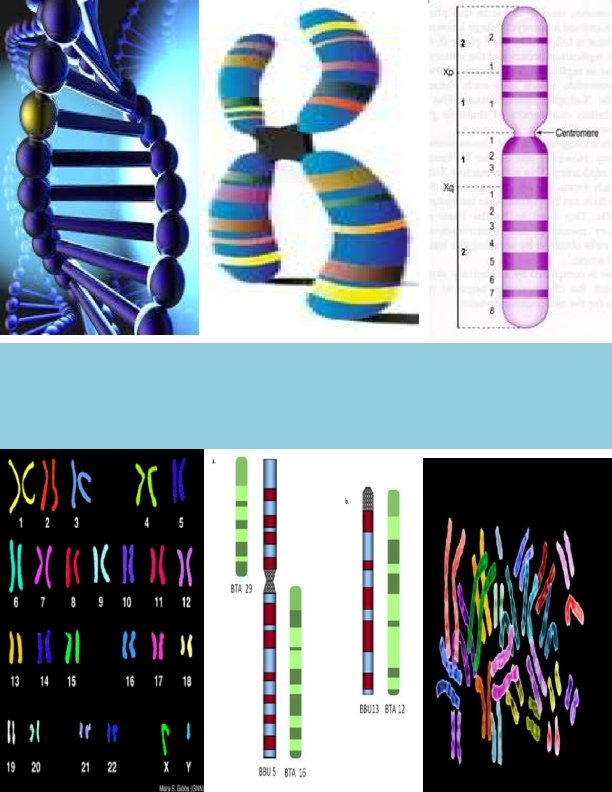
Karyotyping Definition: “It is a process of arranging each pair of homologous chromosomes in a sequence, the longest chromosomes being placed at the beginning and the shortest at the end”. Indications for chromosome analysis: o. Multiple congenital abnormalities o. Unexplained mental retardation o. Sexual ambiguity o. Infertility o. Recurrent miscarriage o. Still birth o. Malignancy & chromosome breakage syndrome 4
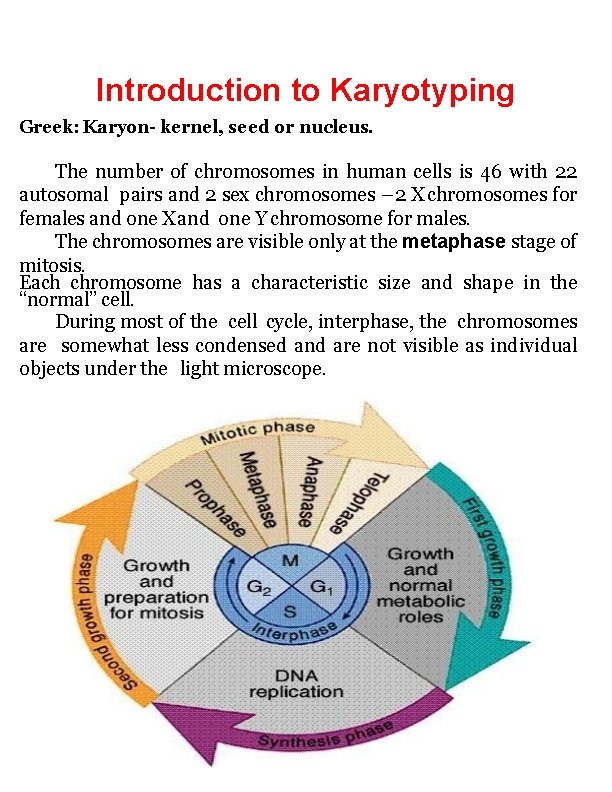
Introduction to Karyotyping Greek: Karyon- kernel, seed or nucleus. The number of chromosomes in human cells is 46 with 22 autosomal pairs and 2 sex chromosomes – 2 X chromosomes for females and one X and one Y chromosome for males. The chromosomes are visible only at the metaphase stage of mitosis. Each chromosome has a characteristic size and shape in the “normal” cell. During most of the cell cycle, interphase, the chromosomes are somewhat less condensed and are not visible as individual objects under the light microscope.
Method: Samples which can be used: Skin Bone marrow Peripheral blood Amniotic fluid (Chorionic villi sampling) Blood culture media preparation: Blood culture media; 500 ml RPMI 1640 with 100 ml fetal bovine serum, 6. 5 ml penicillin – streptomycin and 7 ml glutamine. Dispense 10 ml aliquots into sterile tube and add 2% (0. 2 ml) PHA to each tube. Store at 4°C for as long as 2 weeks. Lymphocyte cells do not normally undergo subsequent cell divisions. In the presence of a mitogen (PHA), lymphocytes are stimulated to enter into mitosis by DNA replication. After 48 -72 hours, a mitotic inhibitor (colcemid) is added to the culture to stop mitosis in the metaphase stage. Other culture media which can be used are TC 199 and HAM F 10. 6
Blood preparation: 1. Wash 10 ml syringe with Heparin solution and collect blood aseptically to the syringe (0. 5 ml for each tube). 2. Remove the blood to sterile vacuette tubes or to sterile, heparin prewashed tube. 3. Store at 4°C until required (upto 7 hours). 4. Before use, invert the tube with blood several times. 7

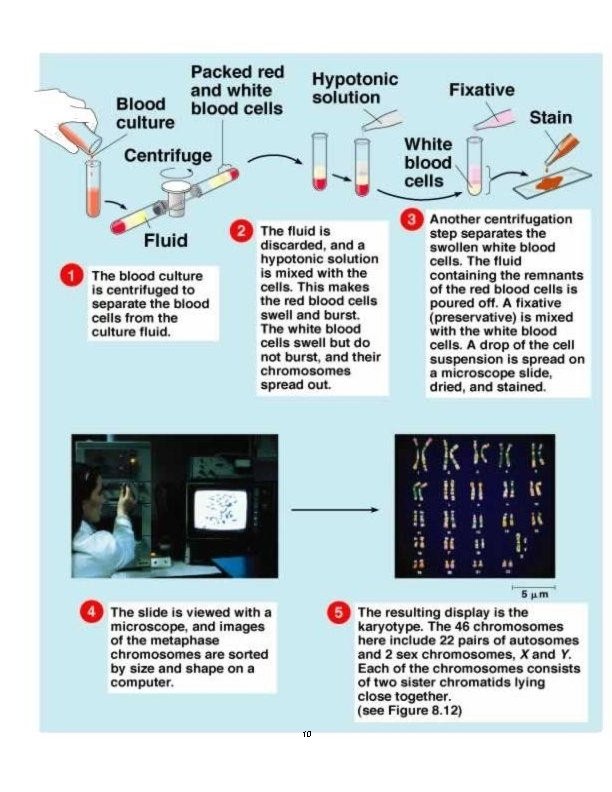
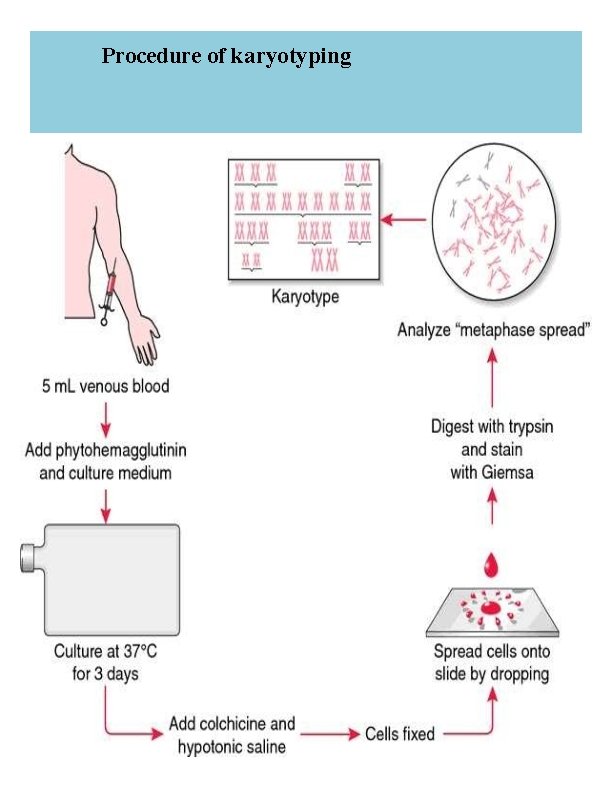
Karyotyping procedure: 1. Inoculate 0. 5 ml of heparinized whole blood into tube with 10 ml of karyotyping medium. 2 Incubate the tubes in incubator with 5% CO 2 at 37°C for total of 69 -72 hours. 3 -After total of 69 hours from seeding add 100μl of Colcemid solution to each culture tubes. 4 - Incubate the tubes at 37°C for an additional 20 -30 minutes. 5 - Spin at 500 g (1500 RPM) for 7 minutes. 6 - Remove the supernatant and re-suspend the cells in 5 ml of hypotonic 0. 075 M KCl pre wormed to 37°C. 7 Incubate at 37°C for 15 minutes. 8 Add drop-by- drop (with vortexing) 1 ml fresh ice cold fixative. 9 - Spin at 500 g (1500 RPM) for 7 minutes. 10 - Remove the supernatant, agitate the cellular sediment and add dropby drop (with continuous vortexing), 5 ml of fresh, ice-cold fixative. 11 - Leave at 4°C for 20 minutes. 12 - Repeat steps 9 and 10, until the supernatant is clear. 13 - Spin at 500 g (1500 RPM) for 7 minutes. 14 Re-suspend the cell pellet with a 1. 5 ml of fresh fixative. 15 Drop 4 -5 drops, from a height of approximately 50 cm onto a clean slide and blow carefully on the drops for spreading them on the slide. 16 Heat the slides to 55°C for overnight.

Staining Procedure: The staining procedure should be done in a 37°C water bath. Put the slides in a staining rack (e. g. coplin staining jar) and treat as follows: 2 minutes in 50 ml Hanks solution. 1. 47 minutes in: 47. 5 ml Hanks solution + 2. 5 ml Trypsin-EDTA solution. Wash in: 40 ml Hanks solution +10 ml Fetal bovine serum. Wash in: 50 ml Hanks solution. Wash 51. 5 minutes in: 47 ml Buffer solution p. H 6. 8 + 3 ml Giemsa stain solution. Wash several times with 50 ml Buffer solution p. H 6. 8 Air dry the chromosome slides. Check for chromosome spreads in a phase contrast lab microscope
10

Karyotype analysis: 1 Obtain a set of chromosomes. 2 Take a photograph of chromosome spread. The photograph is enlarged and cut up into individual chromosomes. 3 The homologous chromosomes are distinguished by leangth and by the position of the centromere. 4 Match the chromosomes with their homologous mate. One Chromosome of each pair is numbered, as you match your chromosomes; number the homologous pairs. You need to be very systematic. The number one chromosome is the largest. Its corresponding mate should be of the same size, with the same banding pattern, and have the same centromere location. There are 22 pairs of chromosomes which match up exactly. Then the sex chromosomes are paired, in the female (XX) and in the male (XY). This technique can be used to assess the “normalcy” of an individual’s chromosomes and to assay for various genetic diseases such as Down’s syndrome and Klinefelter’s syndrome. It is estimated that one in 156 live births have some kind of chromosomal abnormality. 11

5. Karyotypes are arranged with the short arm of the chromosome on top, and the long arm on the bottom. 6. In addition, the differently stained regions and subregions are given numerical designations from proximal to distal on the chromosome arms. For example, Cri du chat syndrome involves a deletion on the short arm of chromosome 5. It is written as 46, XX, 5 p-. The critical region for this syndrome is deletion of 15. 2, which is written as 46, XX, del(5)(p 15. 2).
Procedure of karyotyping

Classification of Chromosomes: Morphological classification Giemsa stain is used to classify the chromosomes only on the basis of their overall morphology. Chromosome consists of centromere and 2 arms labeled as p and q. Short –p-arm (petit) Long –q- arm (q/g grand) Classification By Position of Centromere: 1. Metacentric 2. Submetacentric 3. Acrocentric: Except Y chromosomes, all have satellites attached by secondary constricitions 4. Telocentric The centromeres can be found in the middle of the chromosome (metacentric), near one end (acrocentric), or in between these first two (submetacentric). Telocentric chromosomes are not found in human cells. 14
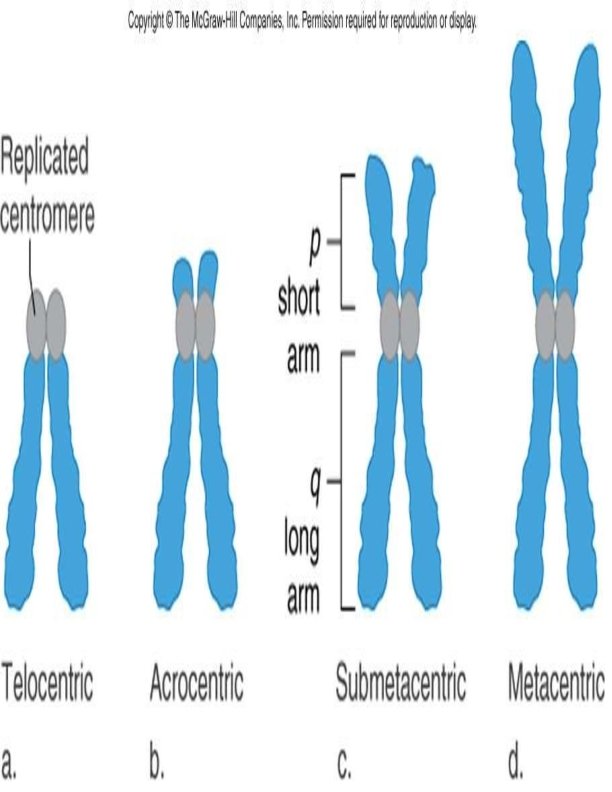

Denver- London system of classification (1960) : Chromosomes are arranged into seven groups based on size and centromere location. Group A: chromosomes 1 -3 are largest with median centromere. Group B: chromosomes 4 -5 are large with submetacentric centromere. Group C: chromosomes 6 -12 are medium sized with submetacentric centromere. Group D: chromosomes 13 -15 are medium sized with acrocentric centromere Group E: chromosomes 16 -18 are fairly short with submetacentric centromere Group F: chromosomes 19 -20 are short with median centromere Group G: chromosomes 21 -22 are very short with acrocentric centromere. Chromosome X is similar to group C (sub-metacentric). Chromosome Y is similar to group G-acrocentric ( without satellite body). 16

Representation of a karyotype v By arranging chromosome of somatic complement in a descending order of size keeping their centromeres in a straight line. Longest chromosome –on extreme left. Shortest chromosome –on extreme right Sex chromosomes –allosomes–extreme right
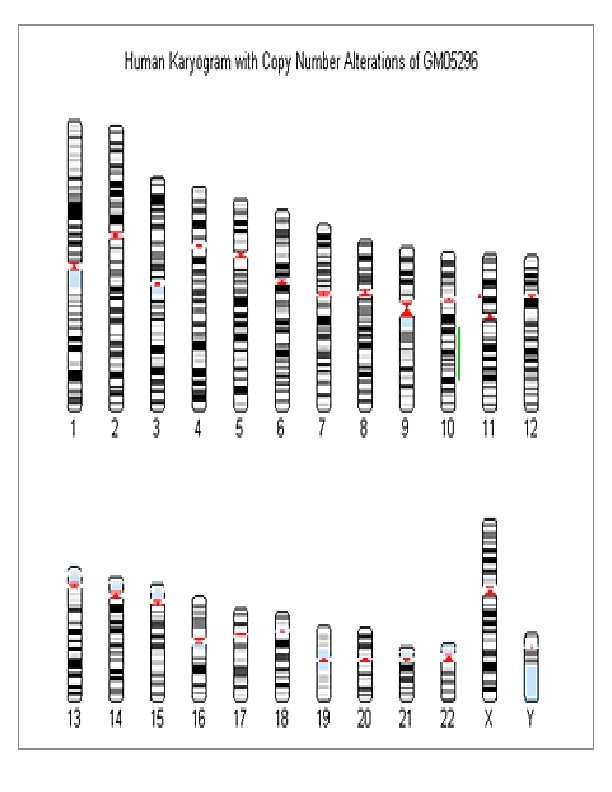
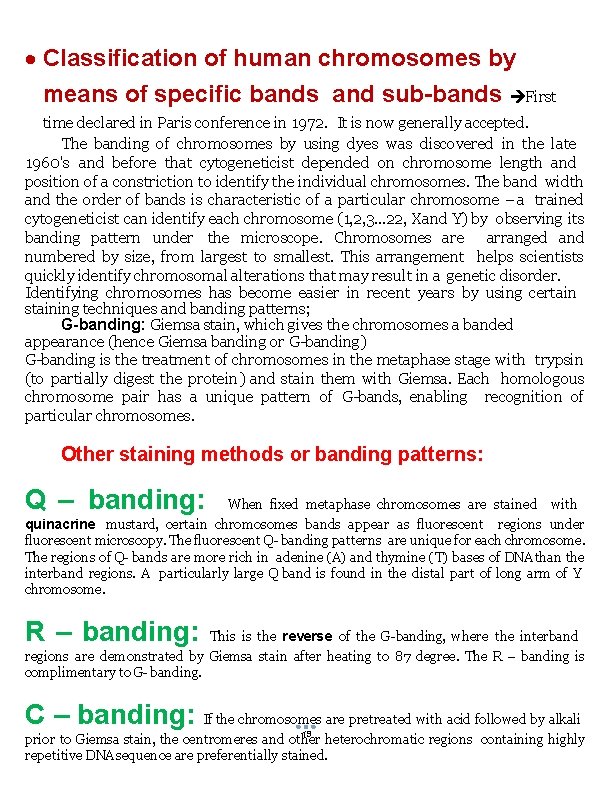
Classification of human chromosomes by means of specific bands and sub-bands First time declared in Paris conference in 1972. It is now generally accepted. The banding of chromosomes by using dyes was discovered in the late 1960's and before that cytogeneticist depended on chromosome length and position of a constriction to identify the individual chromosomes. The band width and the order of bands is characteristic of a particular chromosome – a trained cytogeneticist can identify each chromosome (1, 2, 3. . . 22, Xand Y) by observing its banding pattern under the microscope. Chromosomes are arranged and numbered by size, from largest to smallest. This arrangement helps scientists quickly identify chromosomal alterations that may result in a genetic disorder. Identifying chromosomes has become easier in recent years by using certain staining techniques and banding patterns; G-banding: Giemsa stain, which gives the chromosomes a banded appearance (hence Giemsa banding or G-banding) G-banding is the treatment of chromosomes in the metaphase stage with trypsin (to partially digest the protein) and stain them with Giemsa. Each homologous chromosome pair has a unique pattern of G-bands, enabling recognition of particular chromosomes. Other staining methods or banding patterns: Q – banding: When fixed metaphase chromosomes are stained with quinacrine mustard, certain chromosomes bands appear as fluorescent regions under fluorescent microscopy. The fluorescent Q- banding patterns are unique for each chromosome. The regions of Q- bands are more rich in adenine (A) and thymine (T) bases of DNA than the interband regions. A particularly large Q band is found in the distal part of long arm of Y chromosome. R – banding: This is the reverse of the G-banding, where the interband regions are demonstrated by Giemsa stain after heating to 87 degree. The R – banding is complimentary to G- banding. C – banding: If the chromosomes are pretreated with acid followed by alkali 19 prior to Giemsa stain, the centromeres and other heterochromatic regions containing highly repetitive DNA sequence are preferentially stained.

Advantages of Karyotyping • Reveals structural features of each chromosomes. • Helps in studying chromosome banding pattern. • Helps in the identification of chromosomal aberrations. • Diagnosis of prenatal genetic defects. • Aids in studying evolutionary changes.
Chromosomal Abberation: Definition: Abnormalities of chromosome. Frequency: 6/1000 live birth Major cause of spontaneous abortion, congenital malformations, mental retardation and malignancies. Classification of chromosomal abnormalities 1. Numerical 2. Structural 3. Different Cell lines
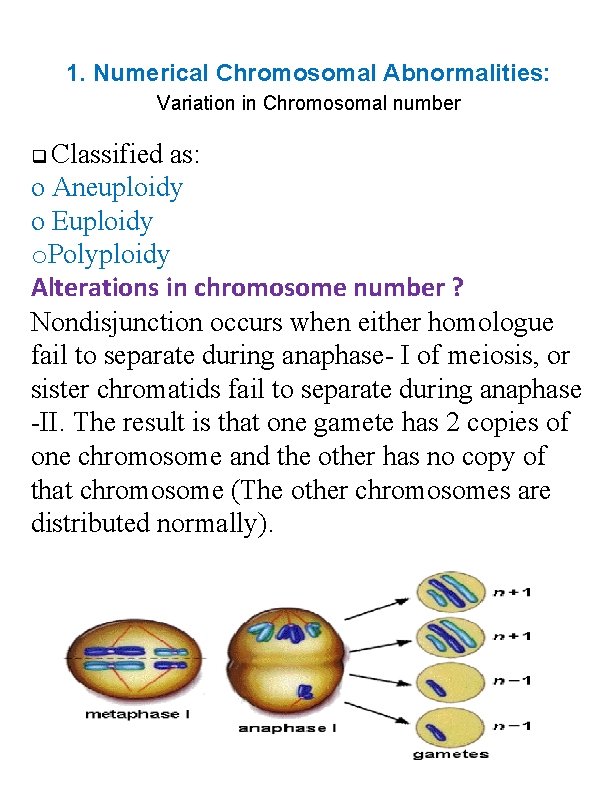
1. Numerical Chromosomal Abnormalities: Variation in Chromosomal number Classified as: o Aneuploidy o Euploidy o. Polyploidy Alterations in chromosome number ? Nondisjunction occurs when either homologue fail to separate during anaphase- I of meiosis, or sister chromatids fail to separate during anaphase -II. The result is that one gamete has 2 copies of one chromosome and the other has no copy of that chromosome (The other chromosomes are distributed normally).

§ If either of these gametes unites with another during fertilization, the result is aneuploidy. A trisomic cell has one extra chromosome (2 n +1) = example: Trisomy 21(Down syndrome), Trisomy 18, Trisomy 13, Klinefelters syndrome (XXY), Superfemale XXX. A Tetrasomy: Extra homologous pair. A monosomic cell has one missing chromosome (2 n - 1) = usually lethal except for one known in humans: Turner's syndrome (monosomy XO). 2. Structural Chromosomal Abnormalities: Alteration in chromosomal structure Causes: Reconstitution in unusual combination following chromosomal breakage. Improperly lining up of chromosome during meiosis. Exposure to teratogen.
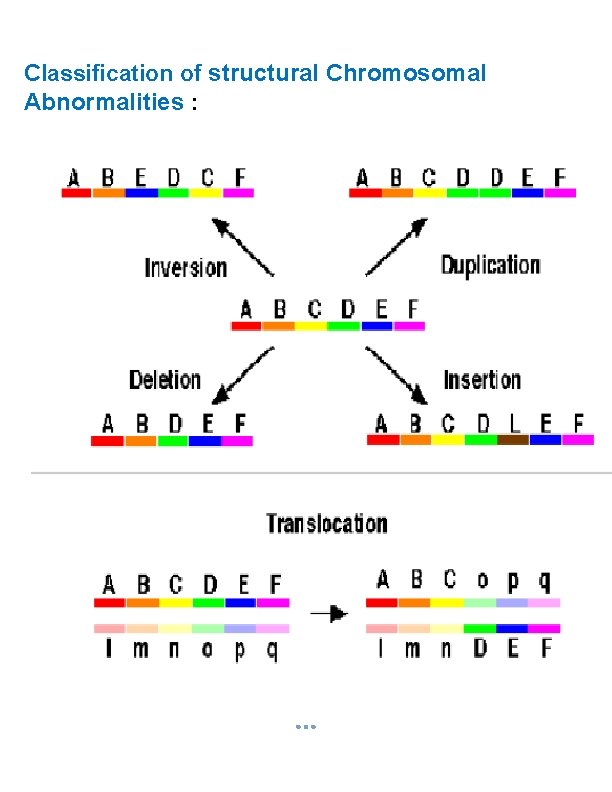
Classification of structural Chromosomal Abnormalities :
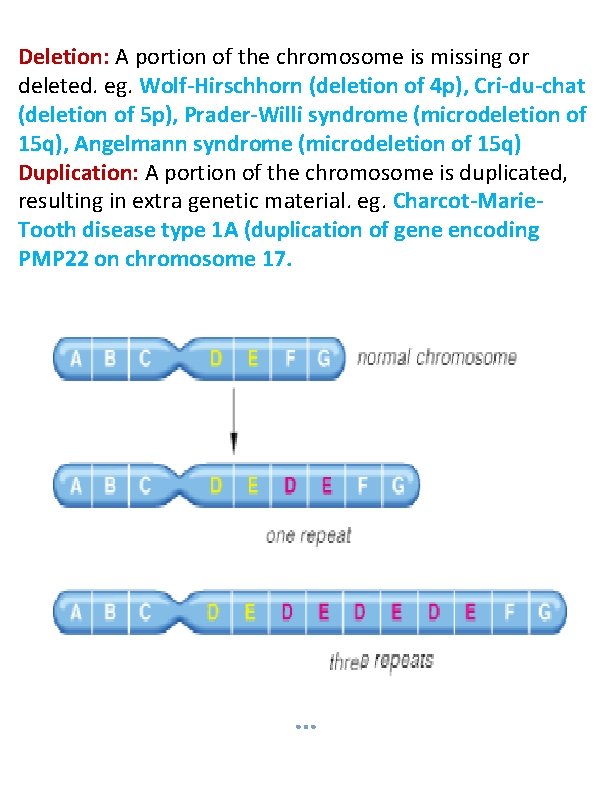
Deletion: A portion of the chromosome is missing or deleted. eg. Wolf-Hirschhorn (deletion of 4 p), Cri-du-chat (deletion of 5 p), Prader-Willi syndrome (microdeletion of 15 q), Angelmann syndrome (microdeletion of 15 q) Duplication: A portion of the chromosome is duplicated, resulting in extra genetic material. eg. Charcot-Marie. Tooth disease type 1 A (duplication of gene encoding PMP 22 on chromosome 17.
Ring Chromosome: A portion of a chromosome has broken off and formed a circle or ring. Isochromosome: Formed by the mirror image copy of a chromosome segment including the centromere. Translocation: When a portion of one chromosome is transferred to another chromosome. There are two main types of translocations. In a reciprocal translocation, segments from two different chromosomes have been exchanged. In a Robertsonian translocation, an entire chromosome has attached to another at the Centromere – in humans these only occur with chromosomes 13, 14, 15, 21 and 22. Inversion: A portion of the chromosome has broken off, turned upside down and reattached, therefore the genetic material is inverted. It may be pericentric when the inversion segment involves the centromere, or paracentric when only one arm of the chromosome is affected.

3. Different cell line Chromosomal Abnormalities: Chimaerism: It is an unusual condition when an individual presents two or more genetically distinct cell lines derived from more than one zygote. In human, two types of chimaeras may be met with- dispermic chimaeras and blood chimaeras. Dispermic chimaeras result from double fertilization whereby two genetically different X-bearing and Ybearing sperms fertilise two ova, and resulting two zygotes fuse to form a single embryo. In such case the chimaeric embryo can develop into an individual with true hermaphroditism with an XX/XY karyotypes. Blood chimaeras appear when the two non-identical twins in utero exchange their cells through the placental barrier. It may so happen that 90% of cells of one twin posseses an XY karyotype with blood group B in the RBC, whereas 90% of cells of the other twin shows an XX karyotype with blood group A in RBCs.
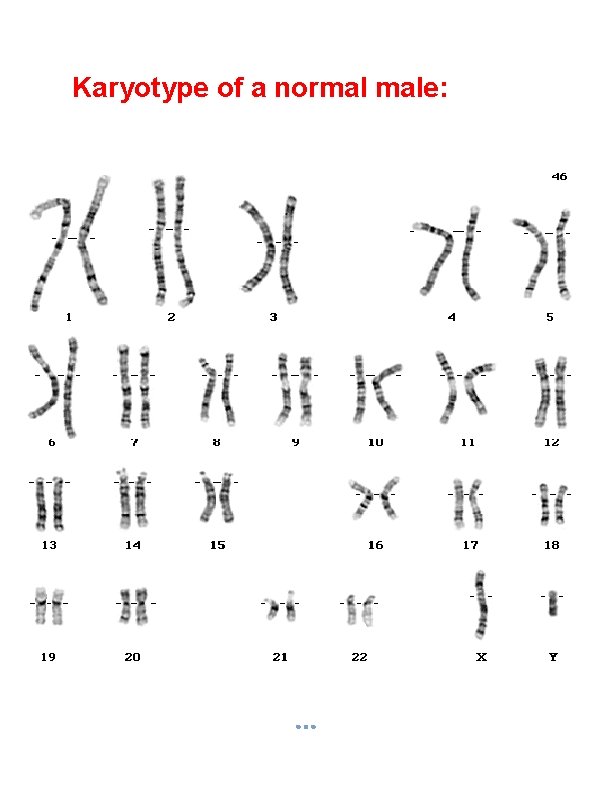
Karyotype of a normal male:
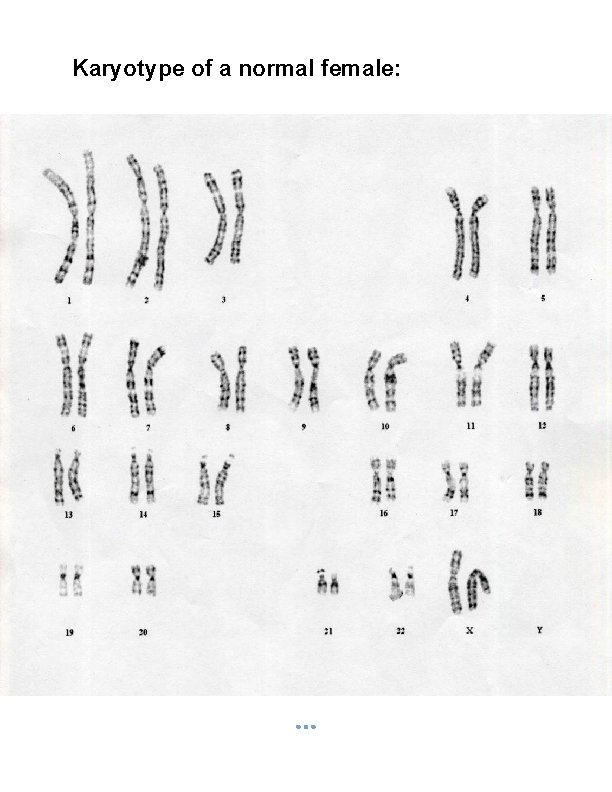
Karyotype of a normal female:
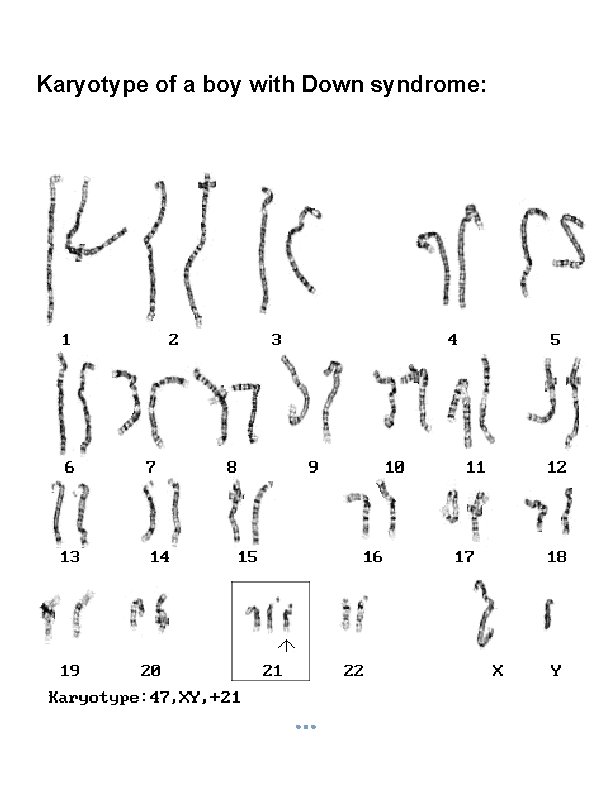
Karyotype of a boy with Down syndrome:
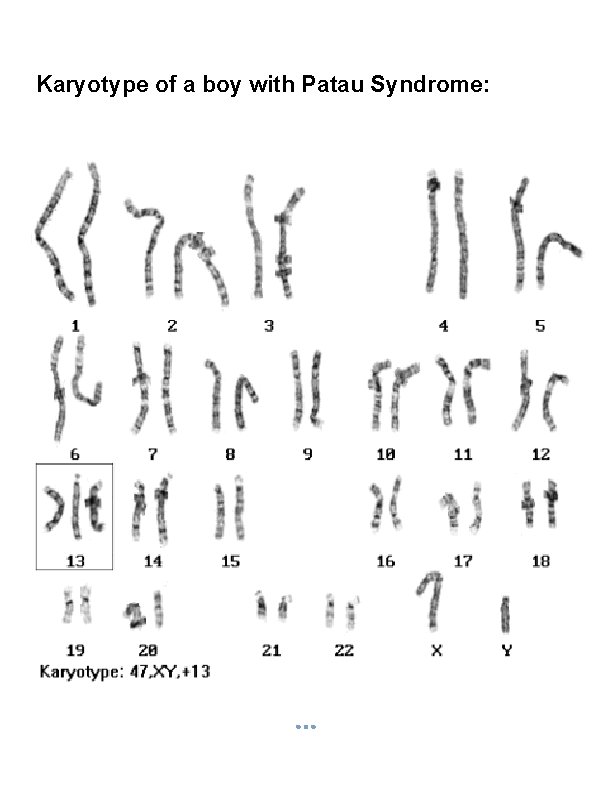
Karyotype of a boy with Patau Syndrome:
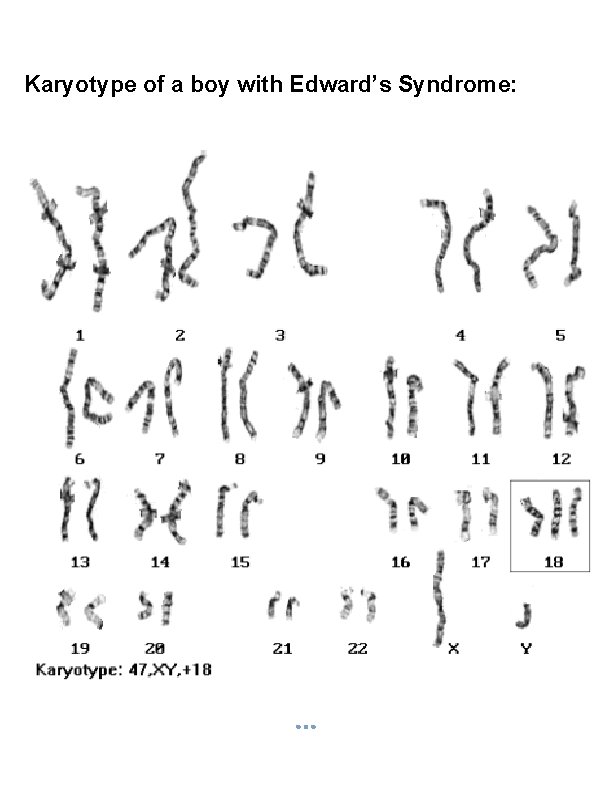
Karyotype of a boy with Edward’s Syndrome:
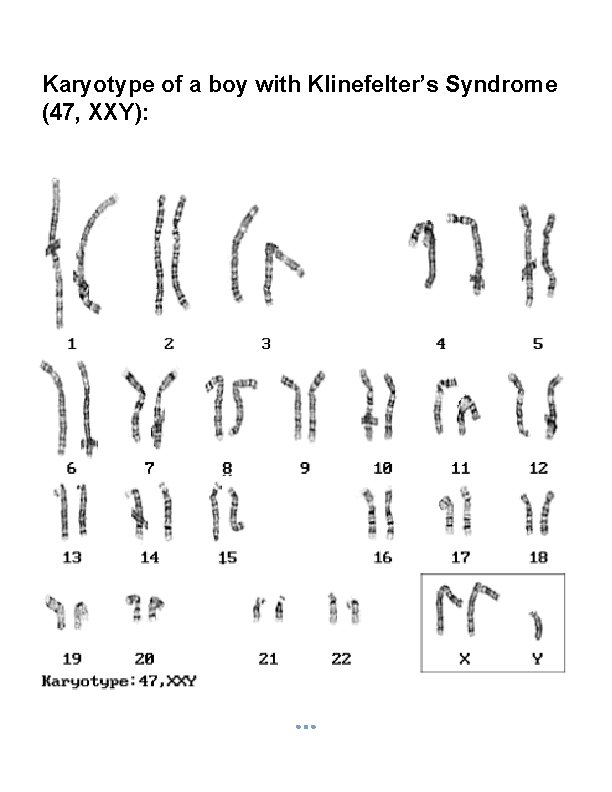
Karyotype of a boy with Klinefelter’s Syndrome (47, XXY):
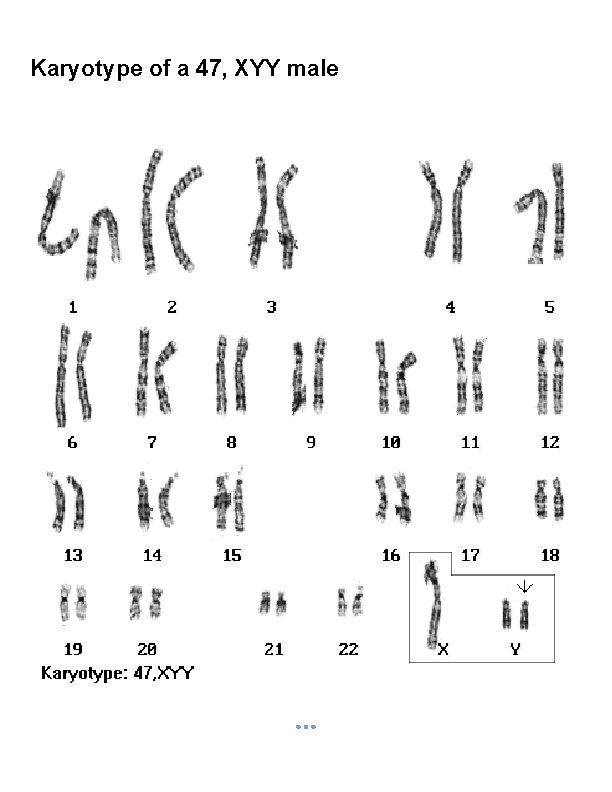
Karyotype of a 47, XYY male
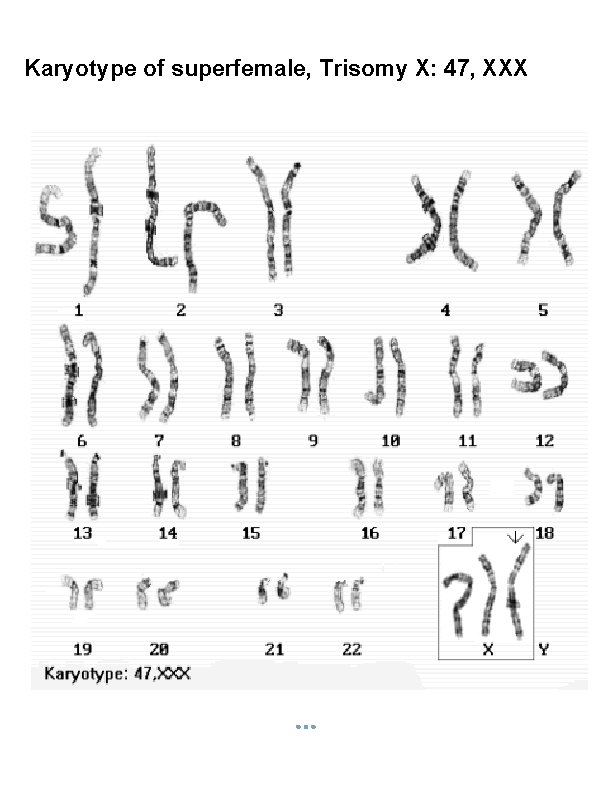
Karyotype of superfemale, Trisomy X: 47, XXX
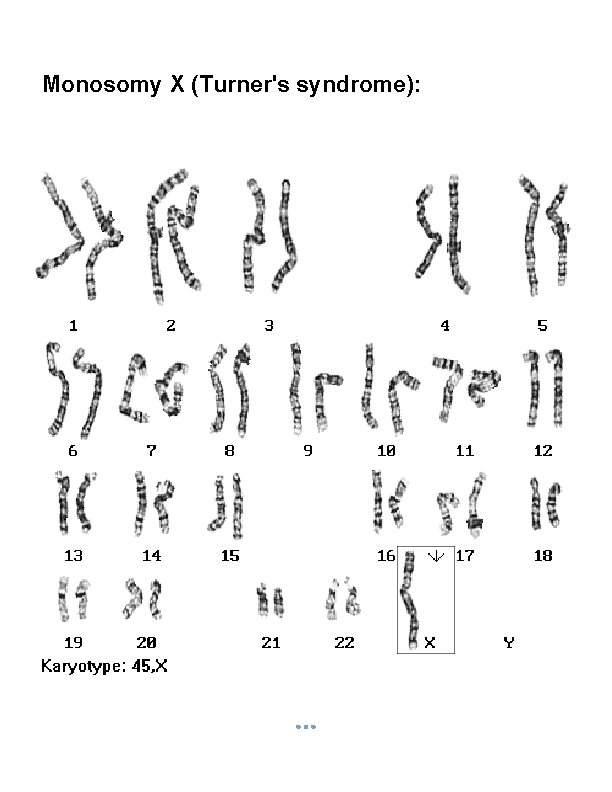
Monosomy X (Turner's syndrome):
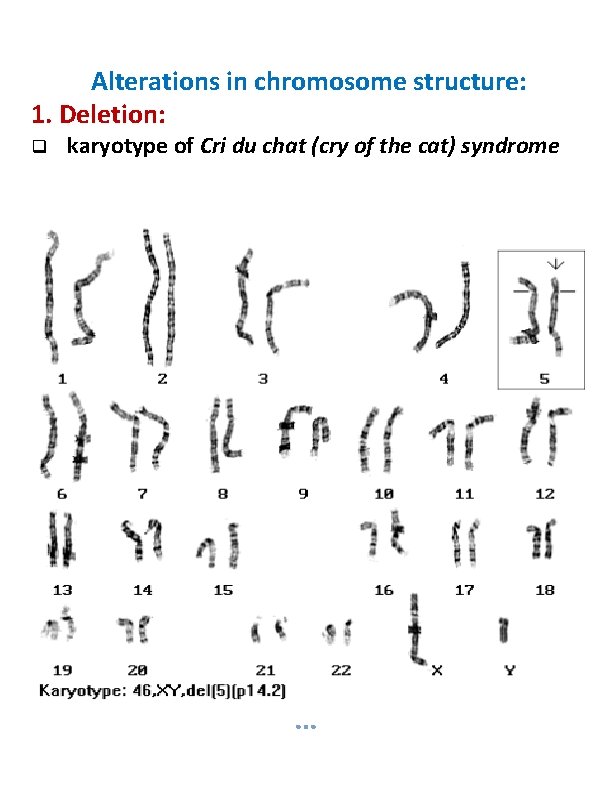
Alterations in chromosome structure: 1. Deletion: karyotype of Cri du chat (cry of the cat) syndrome
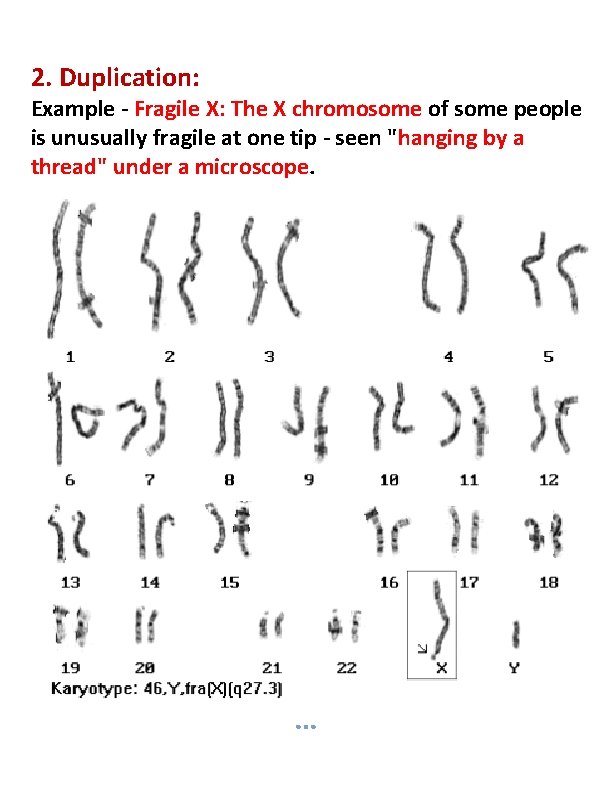
2. Duplication: Example - Fragile X: The X chromosome of some people is unusually fragile at one tip - seen "hanging by a thread" under a microscope.
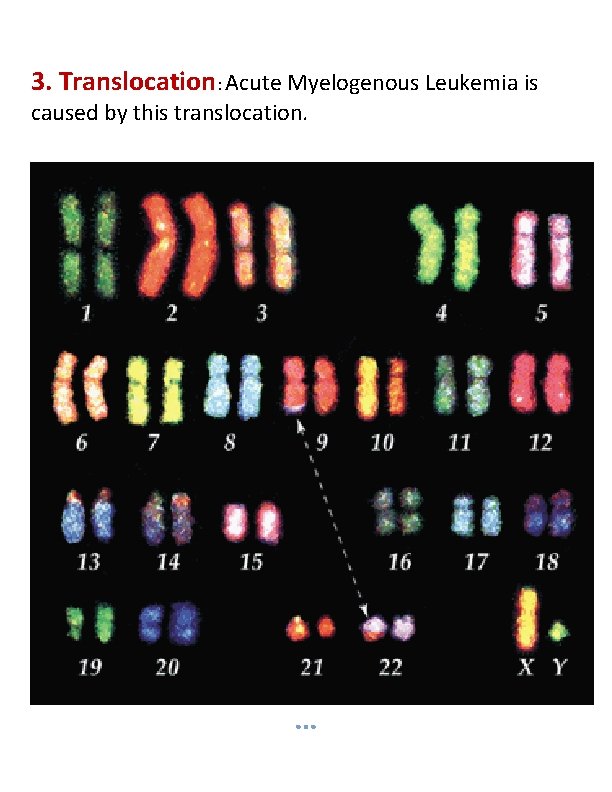
3. Translocation: Acute Myelogenous Leukemia is caused by this translocation.
THANK YOU
- Slides: 40