Week 5 Wednesday Ligation of chromosomal digests into







- Slides: 9

Week 5 • Wednesday: – Ligation of chromosomal digests into plasmid vectors – part II – Checking ligation success • Thursday: – Electrocompetent cell preparation – handout – Test transformation - handout

Ligation of chromosomal digests into plasmid vectors – part II – Checking ligation success • Each of the 4 ligation reactions will be compared to the no-ligase samples prepared previously • Prepare 80 ml of agarose gel and pour as before • Load according to page 87 • The disappearance of the vector band in the ligase reactions indicates success

Week 5 • Thursday: – Electrocompetent cell preparation – handout – Test transformation - handout

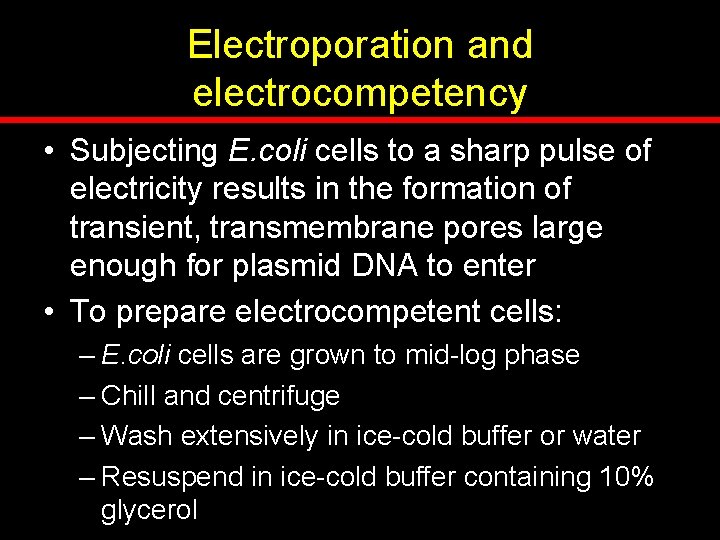
Electroporation and electrocompetency • Subjecting E. coli cells to a sharp pulse of electricity results in the formation of transient, transmembrane pores large enough for plasmid DNA to enter • To prepare electrocompetent cells: – E. coli cells are grown to mid-log phase – Chill and centrifuge – Wash extensively in ice-cold buffer or water – Resuspend in ice-cold buffer containing 10% glycerol

Electrocompetent cell preparation • We will start with a culture inoculated this morning • We will follow the protocol in the handout • We will step through the procedure as a class • It is essential to maintain the cells and all liquids/solutions at 4°C

Test transformation • To test the transformation efficiency of the cells, we will transform 5 ng of plasmid DNA (TA ice bucket) • Procedure is outlined starting with step 12 • The cuvettes must be chilled prior to adding the DNA or the cells

Test Transformation • The plasmid DNA must be dialyzed prior to transformation – drop dialysis – DEMO • Dialyze 5 μl of plasmid DNA as demonstrated • Add 2 μl DNA to the gap; add 40 μl of cells DEMO • Electroporate - DEMO

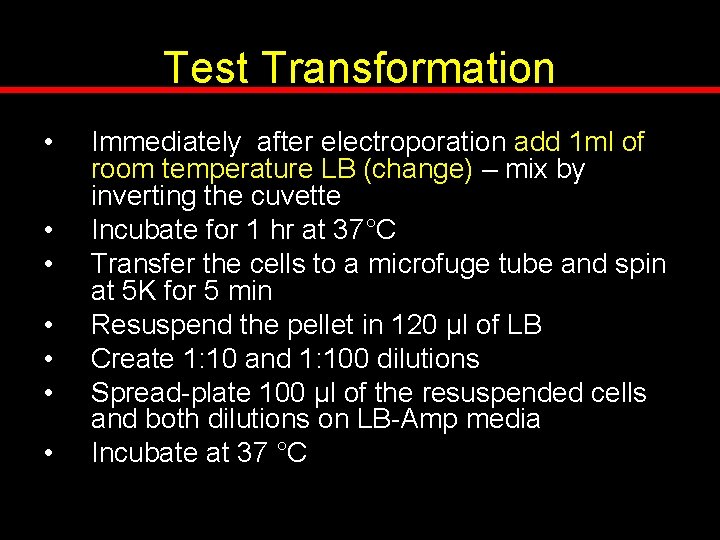
Test Transformation • • Immediately after electroporation add 1 ml of room temperature LB (change) – mix by inverting the cuvette Incubate for 1 hr at 37°C Transfer the cells to a microfuge tube and spin at 5 K for 5 min Resuspend the pellet in 120 μl of LB Create 1: 10 and 1: 100 dilutions Spread-plate 100 μl of the resuspended cells and both dilutions on LB-Amp media Incubate at 37 °C

Due Wednesday Feb 14: • Ex. 9 B – Agarose plate fluorescence quantitation of DNA • Ex. 10, part I & II – Ligation and ligation check experiments