Week 5 Live Cell Imaging in Confocal Microscopy











- Slides: 59

Week 5 Live Cell Imaging in Confocal Microscopy Multiphoton Microscopy Spectral Imaging BME 695 Y / BMS 634 Confocal Microscopy: Techniques and Application Module Purdue University Department of Basic Medical Sciences, School of Veterinary Medicine & Department of Biomedical Engineering, Schools of Engineering These slides are intended for use in a lecture series. Copies of the graphics are distributed and students encouraged to take their notes on these graphics. The intent is to have the student NOT try to reproduce the figures, but to LISTEN and UNDERSTAND the material. All material copyright J. Paul Robinson unless otherwise stated, however, the material may be freely used for lectures, tutorials and workshops. It may not be used for any commercial purpose. A useful text for this course is Pawley “Introduction to Confocal Microscopy”, Plenum Press, 2 nd Ed. A number of the ideas and figures in these lecture notes are taken from this text or of the WEB. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 1 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Lecture Summary • • 1. Live cell confocal microscopy 2. Live cell applications and examples 3. Multiphoton microscopy 4. Spectral Imaging Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 2 t: /classes/BMS 602 B/lecture 5 602_B. ppt

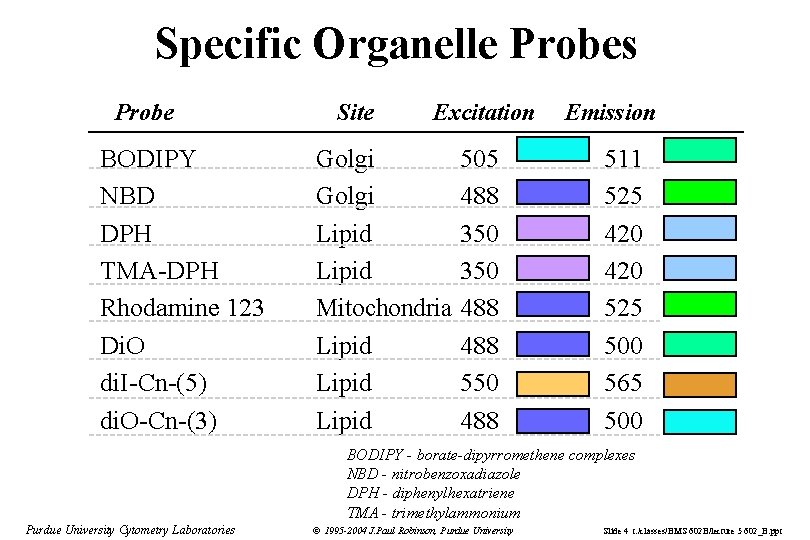
Specific Organelle Probes Probe BODIPY NBD DPH TMA-DPH Rhodamine 123 Di. O di. I-Cn-(5) di. O-Cn-(3) Site Excitation Golgi Lipid Mitochondria Lipid 505 488 350 488 550 488 Emission 511 525 420 525 500 565 500 BODIPY - borate-dipyrromethene complexes NBD - nitrobenzoxadiazole DPH - diphenylhexatriene TMA - trimethylammonium Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 4 t: /classes/BMS 602 B/lecture 5 602_B. ppt

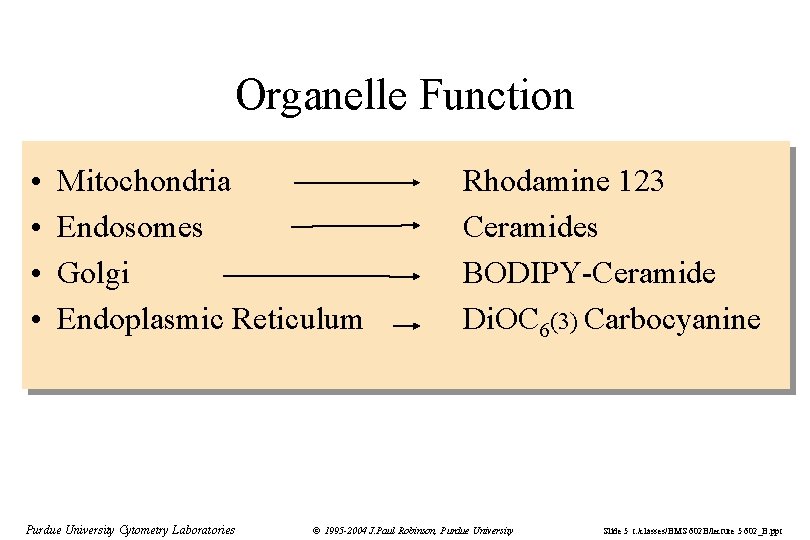
Organelle Function • • Mitochondria Endosomes Golgi Endoplasmic Reticulum Purdue University Cytometry Laboratories Rhodamine 123 Ceramides BODIPY-Ceramide Di. OC 6(3) Carbocyanine © 1995 -2004 J. Paul Robinson, Purdue University Slide 5 t: /classes/BMS 602 B/lecture 5 602_B. ppt

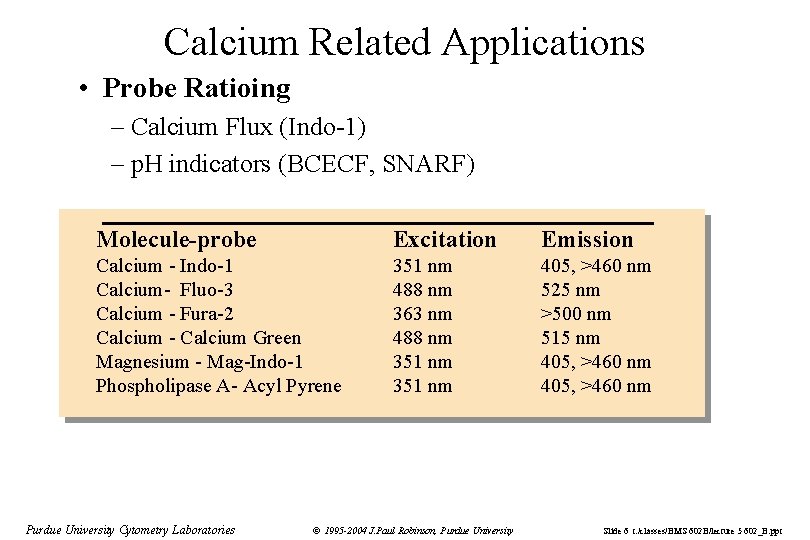
Calcium Related Applications • Probe Ratioing – Calcium Flux (Indo-1) – p. H indicators (BCECF, SNARF) Molecule-probe Excitation Emission Calcium - Indo-1 Calcium- Fluo-3 Calcium - Fura-2 Calcium - Calcium Green Magnesium - Mag-Indo-1 Phospholipase A- Acyl Pyrene 351 nm 488 nm 363 nm 488 nm 351 nm 405, >460 nm 525 nm >500 nm 515 nm 405, >460 nm Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 6 t: /classes/BMS 602 B/lecture 5 602_B. ppt

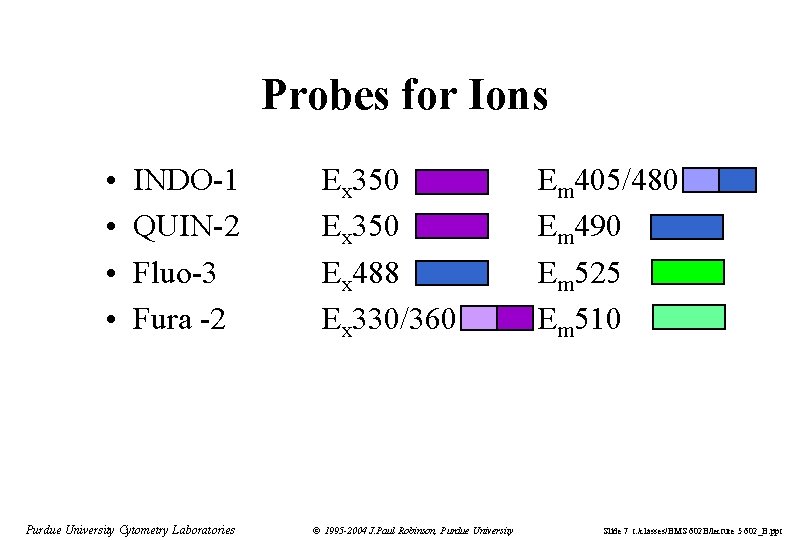
Probes for Ions • • INDO-1 QUIN-2 Fluo-3 Fura -2 Purdue University Cytometry Laboratories Ex 350 Ex 488 Ex 330/360 © 1995 -2004 J. Paul Robinson, Purdue University Em 405/480 Em 490 Em 525 Em 510 Slide 7 t: /classes/BMS 602 B/lecture 5 602_B. ppt

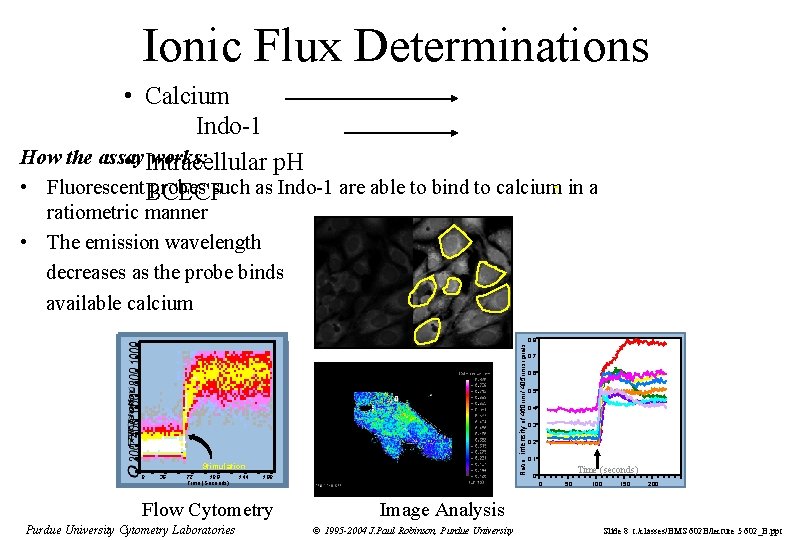
Ionic Flux Determinations • Calcium Indo-1 How the assay works: • Intracellular p. H • Fluorescent. BCECF probes such as Indo-1 are able to bind to calcium in a ratiometric manner • The emission wavelength decreases as the probe binds available calcium Ratio: intensity of 460 nm / 405 nm signals 0. 8 Stimulation 0 36 72 108 Time (Seconds) 144 180 Flow Cytometry Purdue University Cytometry Laboratories 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 Time (seconds) 0 0 50 100 150 200 Image Analysis © 1995 -2004 J. Paul Robinson, Purdue University Slide 8 t: /classes/BMS 602 B/lecture 5 602_B. ppt

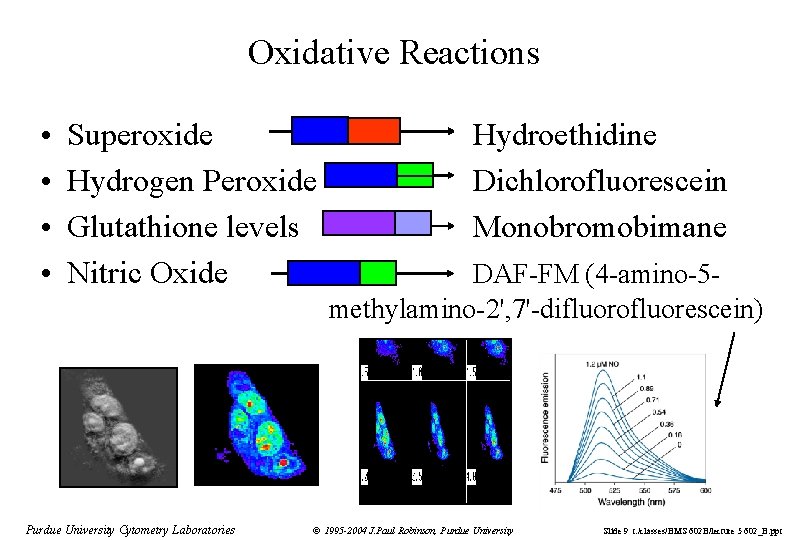
Oxidative Reactions • • Superoxide Hydrogen Peroxide Glutathione levels Nitric Oxide Purdue University Cytometry Laboratories Hydroethidine Dichlorofluorescein Monobromobimane DAF-FM (4 -amino-5 methylamino-2', 7'-difluorofluorescein) © 1995 -2004 J. Paul Robinson, Purdue University Slide 9 t: /classes/BMS 602 B/lecture 5 602_B. ppt

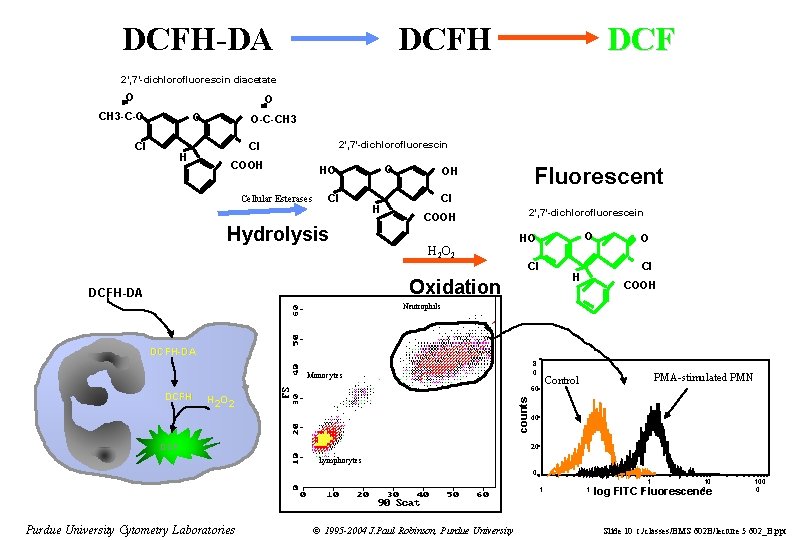
DCFH-DA DCFH DCF 2’, 7’-dichlorofluorescin diacetate O O CH 3 -C-O O Cl H O-C-CH 3 2’, 7’-dichlorofluorescin Cl COOH O HO Cellular Esterases Cl Hydrolysis H Fluorescent OH Cl COOH H 2 O 2 2’, 7’-dichlorofluorescein O HO Cl Oxidation DCFH-DA O Cl H COOH Neutrophils DCFH-DA 8 0 Monocytes H 2 O 2 counts DCFH 60 DCF PMA-stimulated PMN Control 40 20 Lymphocytes 0. 1 Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University 1 1 10 0 0 log FITC Fluorescence 100 0 Slide 10 t: /classes/BMS 602 B/lecture 5 602_B. ppt

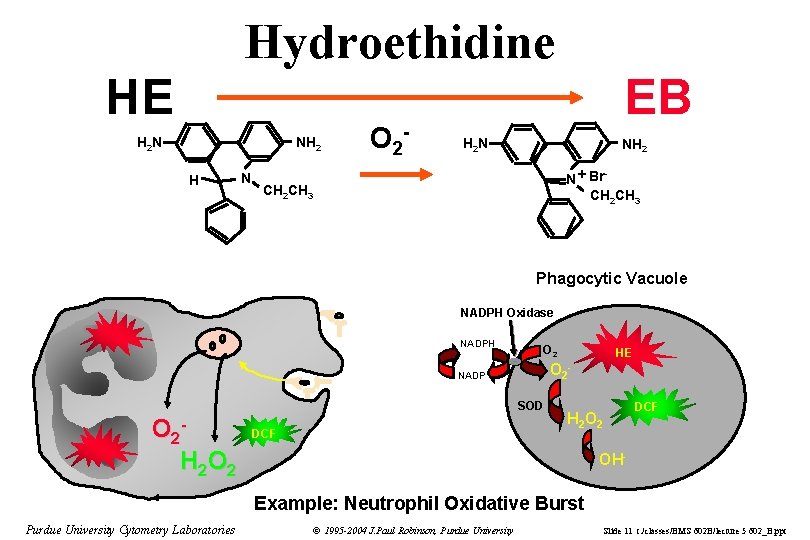
Hydroethidine HE H 2 N NH 2 H N O 2 - EB H 2 N NH 2 N + Br CH 2 CH 3 - CH 2 CH 3 Phagocytic Vacuole NADPH Oxidase NADPH O 2 NADP SOD O 2 H 2 O 2 DCF HE - DCF H 2 OH- Example: Neutrophil Oxidative Burst Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 11 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Macrovascular Endothelial Cells in Culture 0 Purdue University Cytometry Laboratories Time (minutes) © 1995 -2004 J. Paul Robinson, Purdue University 60 Slide 12 t: /classes/BMS 602 B/lecture 5 602_B. ppt

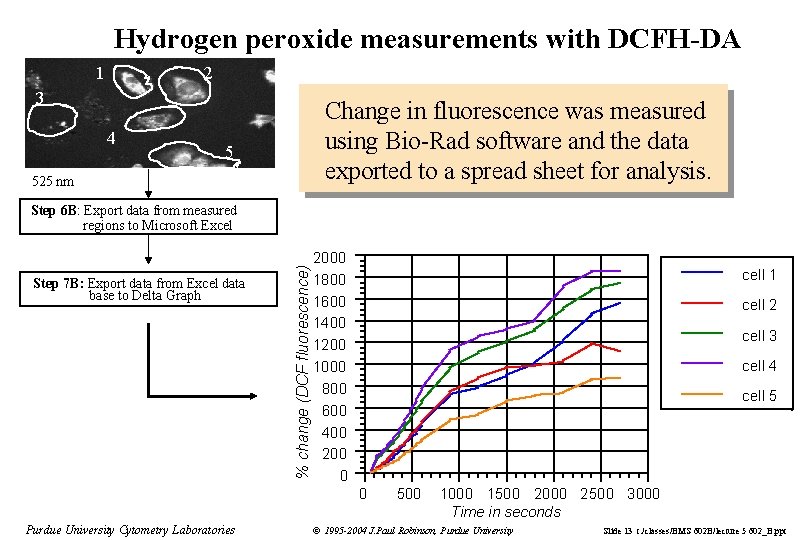
Hydrogen peroxide measurements with DCFH-DA 1 2 3 4 Change in fluorescence was measured using Bio-Rad software and the data exported to a spread sheet for analysis. 5 525 nm Step 7 B: Export data from Excel data base to Delta Graph % change (DCF fluorescence) Step 6 B: Export data from measured regions to Microsoft Excel 2000 1800 1600 1400 1200 1000 800 600 400 200 0 cell 1 cell 2 cell 3 cell 4 cell 5 0 Purdue University Cytometry Laboratories 500 1000 1500 2000 2500 3000 Time in seconds © 1995 -2004 J. Paul Robinson, Purdue University Slide 13 t: /classes/BMS 602 B/lecture 5 602_B. ppt

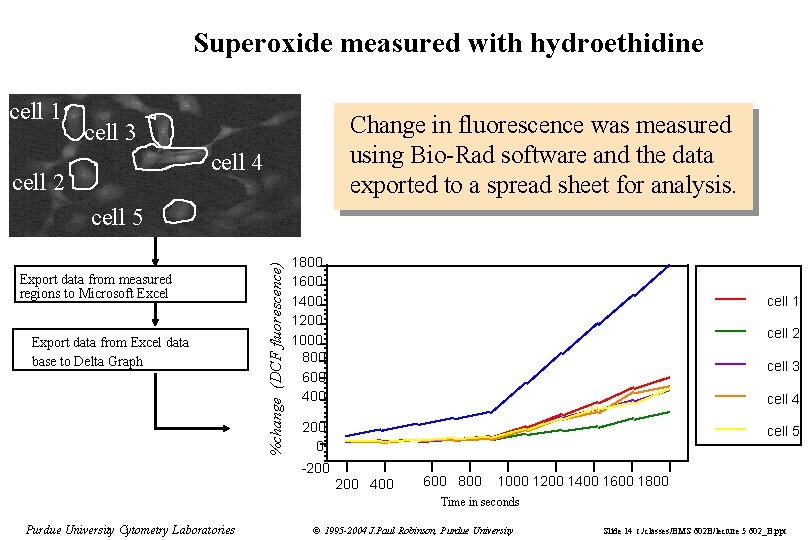
Superoxide measured with hydroethidine cell 1 Change in fluorescence was measured using Bio-Rad software and the data exported to a spread sheet for analysis. cell 3 cell 4 cell 2 Export data from measured regions to Microsoft Excel Export data from Excel data base to Delta Graph %change (DCF fluorescence) cell 5 1800 1600 1400 1200 1000 800 600 400 cell 1 cell 2 cell 3 cell 4 200 0 cell 5 -200 400 600 800 1000 1200 1400 1600 1800 Time in seconds Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 14 t: /classes/BMS 602 B/lecture 5 602_B. ppt

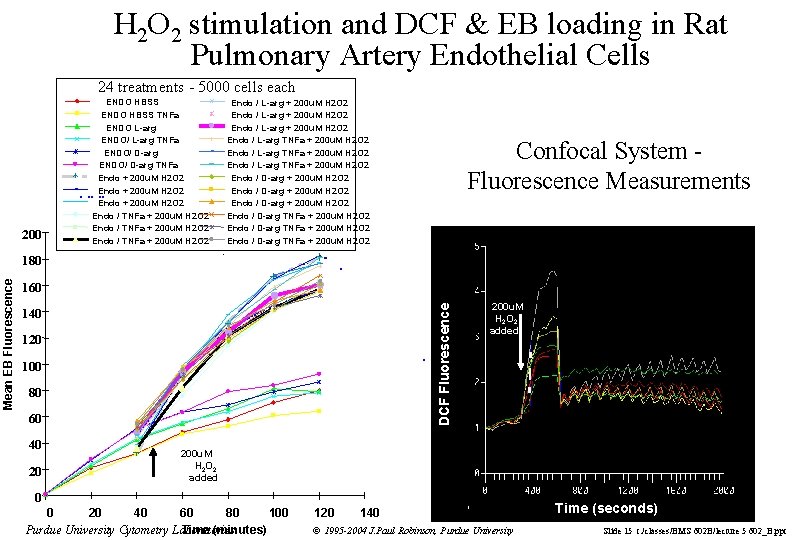
H 2 O 2 stimulation and DCF & EB loading in Rat Pulmonary Artery Endothelial Cells 24 treatments - 5000 cells each 200 ENDO HBSS TNFa ENDO L-arg ENDO/ L-arg TNFa ENDO/ D-arg TNFa Endo + 200 u. M H 2 O 2 Endo / TNFa + 200 u. M H 2 O 2 Confocal System Fluorescence Measurements . 180 160 DCF Fluorescence Mean EB Fluorescence Endo / L-arg + 200 u. M H 2 O 2 Endo / L-arg TNFa + 200 u. M H 2 O 2 Endo / D-arg TNFa + 200 u. M H 2 O 2 140 120 100 80 60 40 20 200 u. M H 2 O 2 added 0 0 20 40 60 80 100 Time (minutes) Purdue University Cytometry Laboratories 120 140 © 1995 -2004 J. Paul Robinson, Purdue University Time (seconds) Slide 15 t: /classes/BMS 602 B/lecture 5 602_B. ppt

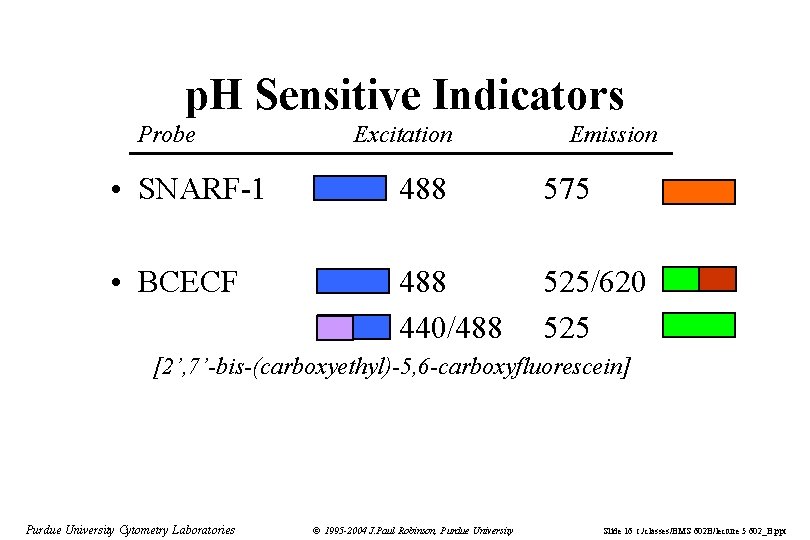
p. H Sensitive Indicators Probe Excitation Emission • SNARF-1 488 575 • BCECF 488 440/488 525/620 525 [2’, 7’-bis-(carboxyethyl)-5, 6 -carboxyfluorescein] Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 16 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Exotic Applications of Confocal Microscopy • • FRAP (Fluorescence Recovery After Photobleaching) Release of “Caged” compounds Lipid Peroxidation (Parinaric Acid) Membrane Fluidity (DPH) Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 17 t: /classes/BMS 602 B/lecture 5 602_B. ppt

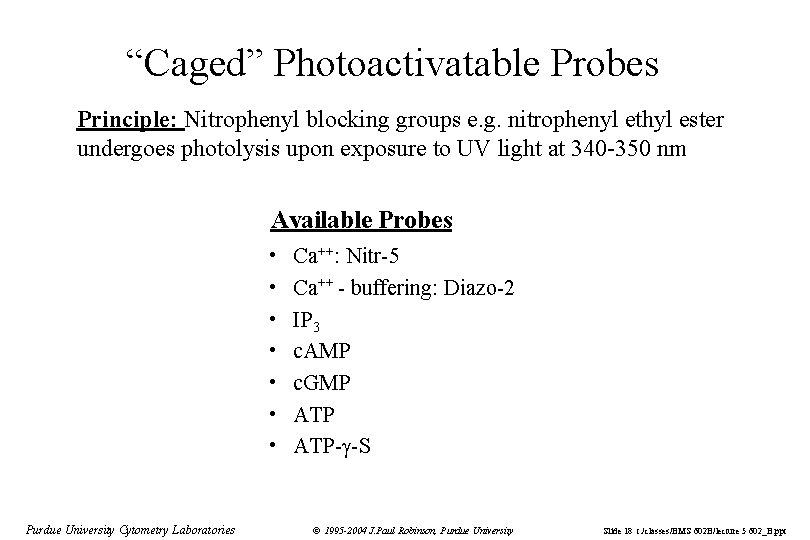
“Caged” Photoactivatable Probes Principle: Nitrophenyl blocking groups e. g. nitrophenyl ethyl ester undergoes photolysis upon exposure to UV light at 340 -350 nm Available Probes • • Purdue University Cytometry Laboratories Ca++: Nitr-5 Ca++ - buffering: Diazo-2 IP 3 c. AMP c. GMP ATP- -S © 1995 -2004 J. Paul Robinson, Purdue University Slide 18 t: /classes/BMS 602 B/lecture 5 602_B. ppt

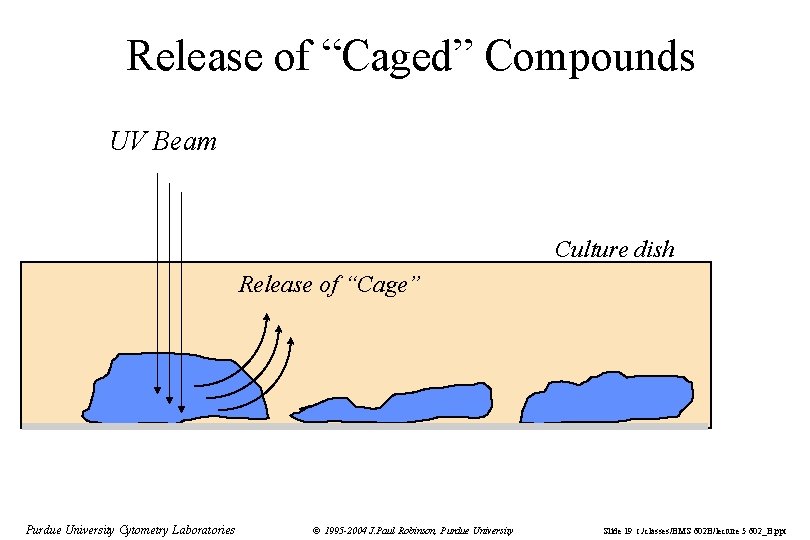
Release of “Caged” Compounds UV Beam Culture dish Release of “Cage” Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 19 t: /classes/BMS 602 B/lecture 5 602_B. ppt

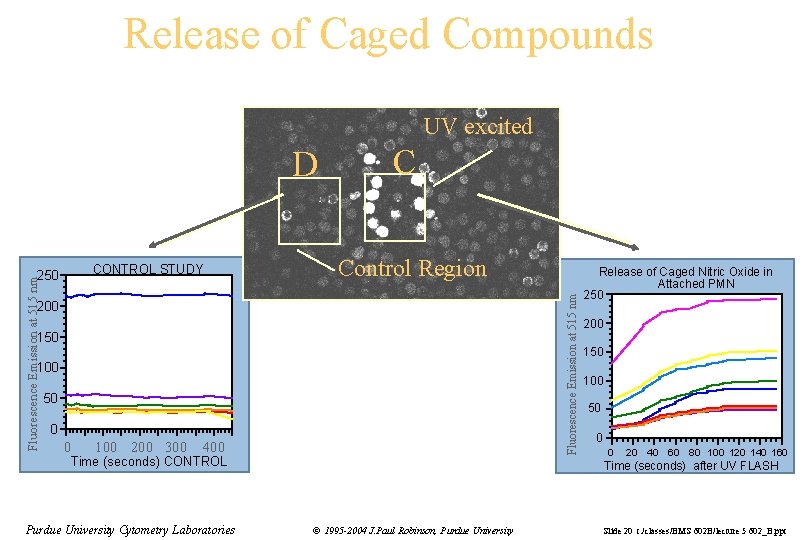
Release of Caged Compounds UV excited D Fluorescence Emission at 515 nm Control Region Fluorescence Emission at 515 nm CONTROL STUDY 250 C 200 150 100 50 0 0 100 200 300 400 Time (seconds) CONTROL Purdue University Cytometry Laboratories Release of Caged Nitric Oxide in Attached PMN 250 200 150 100 50 0 0 20 40 60 80 100 120 140 160 Time (seconds) after UV FLASH © 1995 -2004 J. Paul Robinson, Purdue University Slide 20 t: /classes/BMS 602 B/lecture 5 602_B. ppt

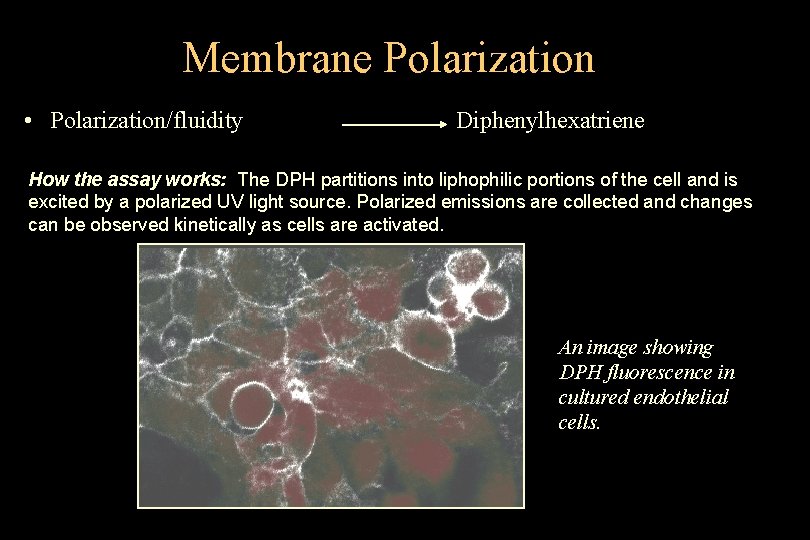
Membrane Polarization • Polarization/fluidity Diphenylhexatriene How the assay works: The DPH partitions into liphophilic portions of the cell and is excited by a polarized UV light source. Polarized emissions are collected and changes can be observed kinetically as cells are activated. An image showing DPH fluorescence in cultured endothelial cells. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 21 t: /classes/BMS 602 B/lecture 5 602_B. ppt

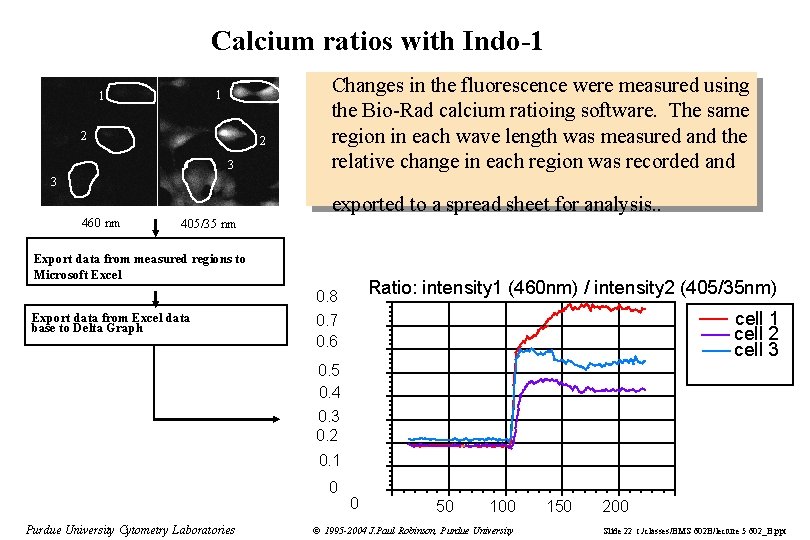
Calcium ratios with Indo-1 1 1 2 2 3 Changes in the fluorescence were measured using the Bio-Rad calcium ratioing software. The same region in each wave length was measured and the relative change in each region was recorded and 3 460 nm exported to a spread sheet for analysis. . 405/35 nm Export data from measured regions to Microsoft Excel Ratio: intensity 1 (460 nm) / intensity 2 (405/35 nm) 0. 8 Export data from Excel data base to Delta Graph cell 1 cell 2 cell 3 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 Purdue University Cytometry Laboratories 0 50 100 © 1995 -2004 J. Paul Robinson, Purdue University 150 200 Slide 22 t: /classes/BMS 602 B/lecture 5 602_B. ppt

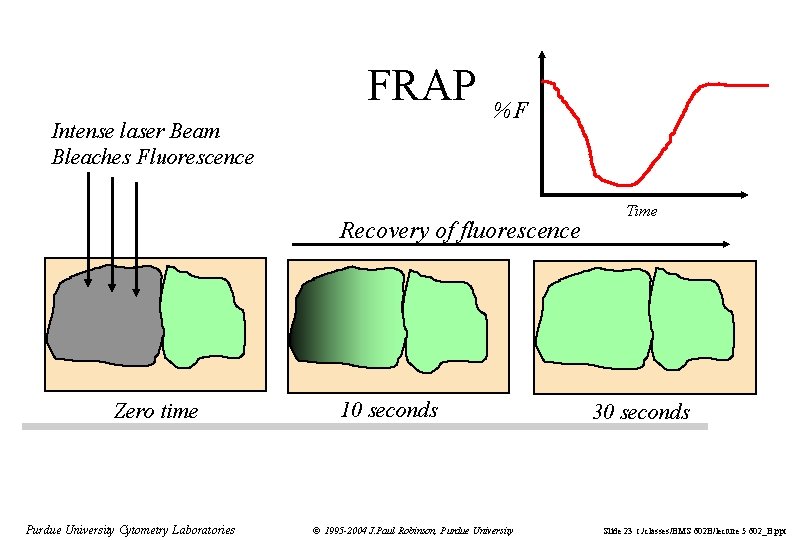
FRAP Intense laser Beam Bleaches Fluorescence %F Recovery of fluorescence Zero time Purdue University Cytometry Laboratories 10 seconds © 1995 -2004 J. Paul Robinson, Purdue University Time 30 seconds Slide 23 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Imaging 3 D ECM structures • Mainly collagen based materials • Usually 40 -120 microns thick • Require both transmitted and fluorescent signals • Often require significant image processing to extract information Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 24 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 25 t: /classes/BMS 602 B/lecture 5 602_B. ppt

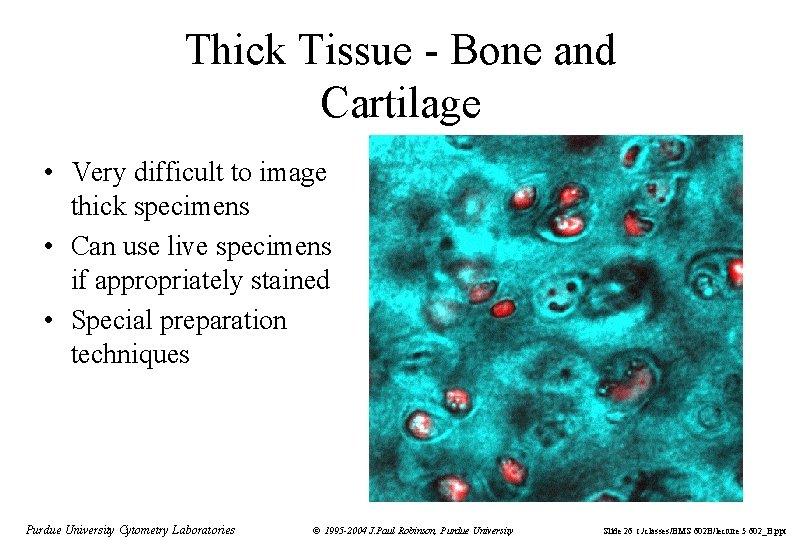
Thick Tissue - Bone and Cartilage • Very difficult to image thick specimens • Can use live specimens if appropriately stained • Special preparation techniques Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 26 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Multi-Photon Microscopy An introduction Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 27 t: /classes/BMS 602 B/lecture 5 602_B. ppt

History • Developed in 1961 by Kaiser and Garret • A process unknown in Nature except in stars • Can be reproduced in a laser beam whereby more than one photon can be absorbed by a molecule in a short time • The energy of both photons is summed in a way similar to that of a photon of shorter wavelength, but the emission is almost identical to that of a single photon Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 28 t: /classes/BMS 602 B/lecture 5 602_B. ppt

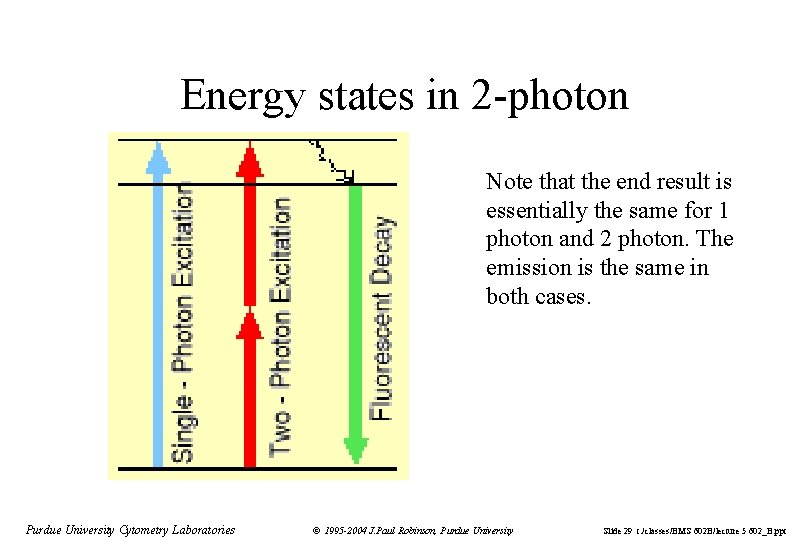
Energy states in 2 -photon Note that the end result is essentially the same for 1 photon and 2 photon. The emission is the same in both cases. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 29 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Advantages of 2 Photon · Longer observation times for live cell studies · Increased fluorescence emission detection · Reduced volume of photobleaching and phototoxicity. Only the focal-plane being imaged is excited, compared to the whole sample in the case of confocal or wide-field imaging. · Reduced autofluorescence of samples · Optical sections may be obtained from deeper within a tissue that can be achieved by confocal or wide-field imaging. There are three main reasons for this: the excitation source is not attenuated by absorption by fluorochrome above the plane of focus; the longer excitation wavelengths used suffer less Raleigh scattering; and the fluorescence signal is not degraded by scattering from within the sample as it is not imaged. · All the emitted photons from multi-photon excitation can be used for imaging (in principle) therefore no confocal blocking apertures have to be used. · It is possible to excite UV flourophores using a lens that is not corrected for UV as these wavelengths never have to pass through the lens. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 30 t: /classes/BMS 602 B/lecture 5 602_B. ppt

2 -Photon Excitation • • The sample is illuminated with a wavelength of twice the wavelength of the absorption peak of the fluorochrome being used. For example, in the case of fluorescein which has an absorption peak around 500 nm, 1000 nm excitation could be used. Essentially no excitation of the fluorochrome will occur at this wavelength and hence no bleaching will occur in the bulk of the sample. A high-powered pulsed laser is required with has a peak power of >2 Kw Power should be in pulses shorter than a picosecond (so that the mean power levels are moderate and do not damage the specimen) Two-photon events will occur at the point of focus give above conditions The photon density is sufficiently high that two photons can be absorbed by the fluorochrome essentially simultaneously. This is equivalent to a single photon with an energy equal to the sum of the two that are absorbed. Thus, fluorochrome excitation will only occur at the point of focus This eliminates unnecessary phototoxicity as there is little excitation out of the plane of focus Image quality is excellent as there is practically no out-of-focus interference. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 31 t: /classes/BMS 602 B/lecture 5 602_B. ppt

3 -Photon Microscopy Three-photon excitation can also be used in certain circumstances. In this case three photons are absorbed simultaneously, effectively tripling the excitation energy. Using this technique, UV excited fluorophores may be imaged with IR excitation. Because excitation levels are dependent on the cube of the excitation power, resolution is improved compared to two photon excitation where there is a quadratic power dependence. It is possible to select fluorophores such that multiple labeled samples by can be imaged by combination of 2 - and 3 photon excitation, using a single IR excitation source. Advantages · UV fluorophore excitation without UV irradiation · Similar resolution to 2 photon excitation of UV fluorophores Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 32 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Limitations of 2 -Photon · Slightly lower resolution with a given fluorochrome when compared to confocal imaging. This loss in resolution can be eliminated by the use of a confocal aperture at the expense of a loss in signal. · Thermal damage can occur in a specimen if it contains chromophores that absorb the excitation wavelengths, such as the pigment melanin. · Only works with fluorescence imaging. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 33 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Why 2 -photon is very specific • Fluorescence from the two-photon effect depends on the square of the incident light intensity, which in turn decreases approximately as the square of the distance from the focus. • Because of this highly nonlinear (~fourth power) behavior, only those dye molecules very near the focus of the beam are excited. • The tissue above and below the plane of focus is merely subjected to infrared light that causes neither photobleaching nor phototoxicity. • Although the peak amplitude of the IR pulses is large, the mean power of the beam is only a few tens of milliwatts, not enough to cause substantial heating of the specimen. Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 34 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Multi-Photon Fluorescence Microscopy The experimental benefits of multi-photon excitation: • Localized excitation provides high spatial resolution • Inherent z-axis resolution improves sensitivity and three-dimensional optical sectioning • Reduced photodamage/ photobleaching • Increased penetration depth in specimen • Provides selective excitation of fluorophores by two and three photons • Increased detection sensitivity of fluorophores by reducing autofluorescence or background • Elimination of confocal aperture Applications for multi-photon microscopy are: • In-vivo and in-vitro imaging • Fluorescent Lifetime Imaging • Optical Tomography Imaging • Semiconductor Imaging http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 35 t: /classes/BMS 602 B/lecture 5 602_B. ppt

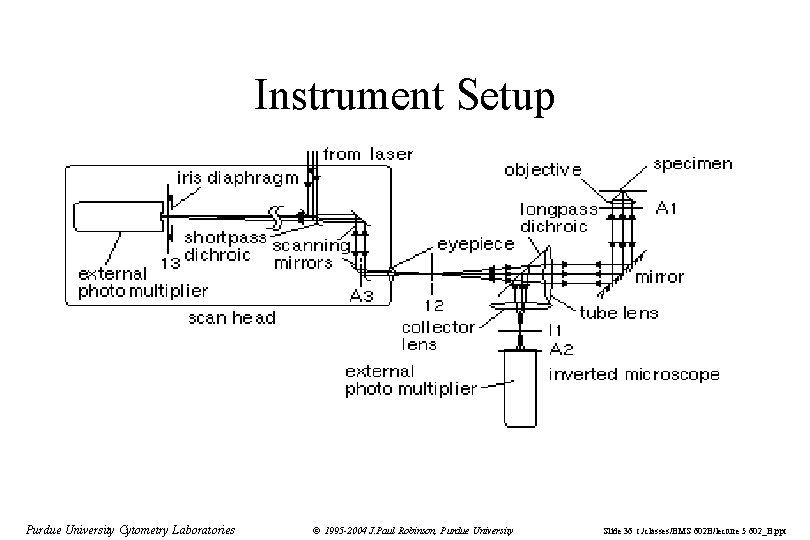
Instrument Setup Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 36 t: /classes/BMS 602 B/lecture 5 602_B. ppt

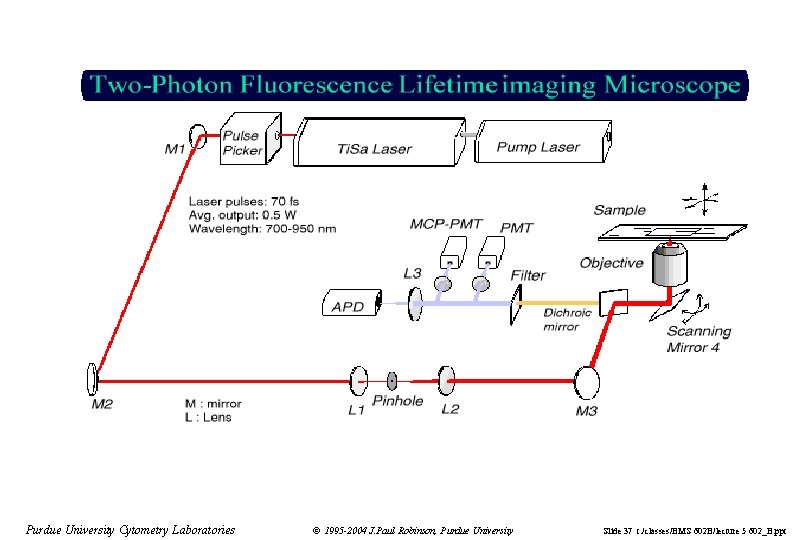
Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 37 t: /classes/BMS 602 B/lecture 5 602_B. ppt

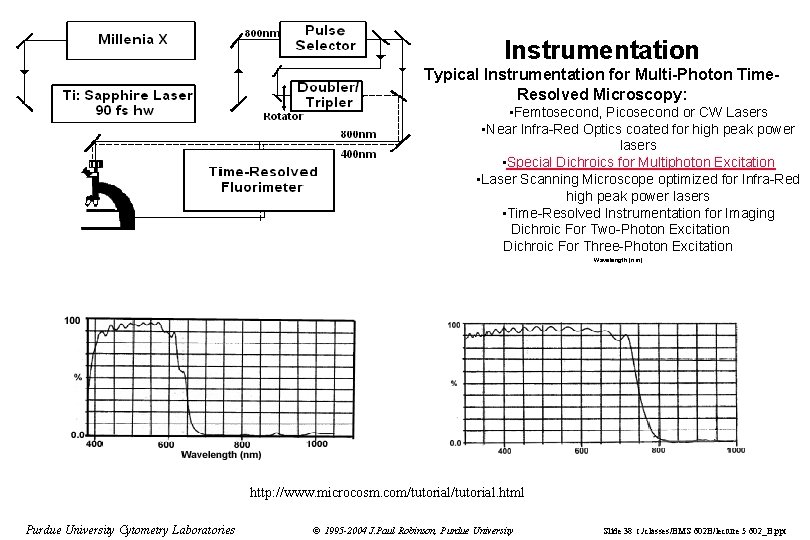
Instrumentation Typical Instrumentation for Multi-Photon Time. Resolved Microscopy: • Femtosecond, Picosecond or CW Lasers • Near Infra-Red Optics coated for high peak power lasers • Special Dichroics for Multiphoton Excitation • Laser Scanning Microscope optimized for Infra-Red high peak power lasers • Time-Resolved Instrumentation for Imaging Dichroic For Two-Photon Excitation Dichroic For Three-Photon Excitation Wavelength (nm) http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 38 t: /classes/BMS 602 B/lecture 5 602_B. ppt

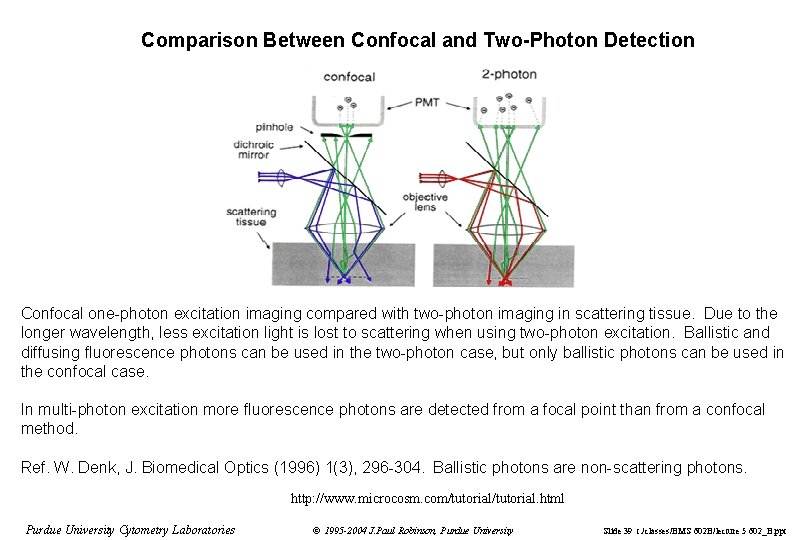
Comparison Between Confocal and Two-Photon Detection Confocal one-photon excitation imaging compared with two-photon imaging in scattering tissue. Due to the longer wavelength, less excitation light is lost to scattering when using two-photon excitation. Ballistic and diffusing fluorescence photons can be used in the two-photon case, but only ballistic photons can be used in the confocal case. In multi-photon excitation more fluorescence photons are detected from a focal point than from a confocal method. Ref. W. Denk, J. Biomedical Optics (1996) 1(3), 296 -304. Ballistic photons are non-scattering photons. http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 39 t: /classes/BMS 602 B/lecture 5 602_B. ppt

2 -photon Vs single photon (confocal) The cuvette is filled with a solution of a dye, safranin O, which normally requires green light for excitation. Green light (543 nm) from a continuous-wave helium-neon laser is focused into the cuvette by the lens at upper right. It shows the expected pattern of a continuous cone, brightest near the focus and attenuated to the left. The lens at the lower left focuses an invisible 1046 -nm infrared beam from a mode-locked Nddoped yttrium lanthanum fluoride laser into the cuvette. Because of the two-photon absorption, excitation is confined to a tiny bright spot in the middle of the cuvette. From Current Protocols in Cytometry Online Copyright © 1999 John Wiley & Sons, Inc. All rights reserved. Purdue University Cytometry Laboratories Photo from Brad Amos © 1995 -2004 J. Paul Robinson, Purdue University Slide 40 t: /classes/BMS 602 B/lecture 5 602_B. ppt

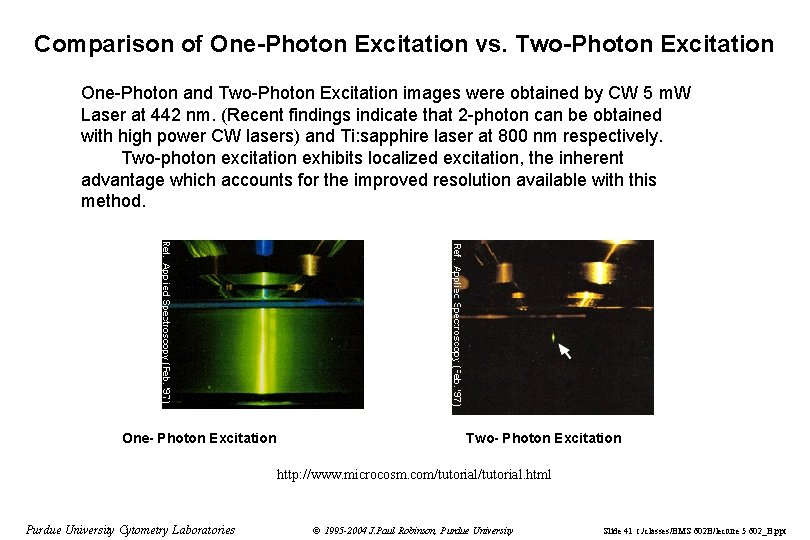
Comparison of One-Photon Excitation vs. Two-Photon Excitation One-Photon and Two-Photon Excitation images were obtained by CW 5 m. W Laser at 442 nm. (Recent findings indicate that 2 -photon can be obtained with high power CW lasers) and Ti: sapphire laser at 800 nm respectively. Two-photon excitation exhibits localized excitation, the inherent advantage which accounts for the improved resolution available with this method. One- Photon Excitation Two- Photon Excitation http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 41 t: /classes/BMS 602 B/lecture 5 602_B. ppt

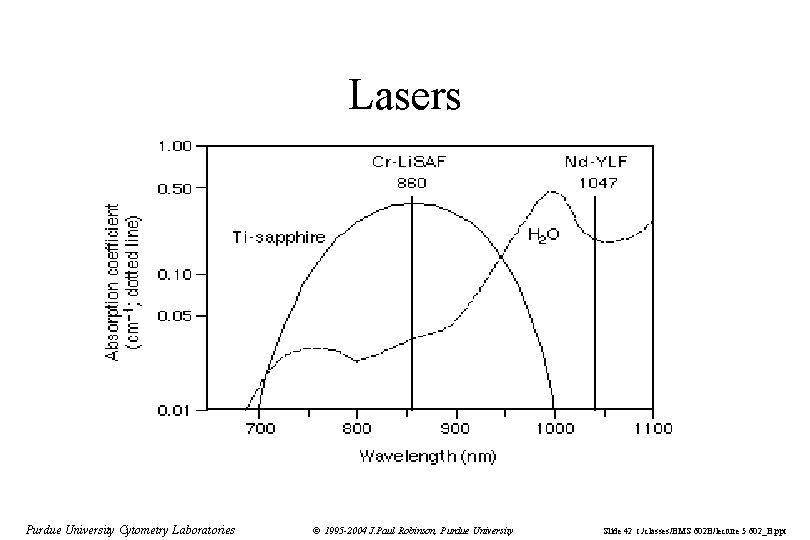
Lasers Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 42 t: /classes/BMS 602 B/lecture 5 602_B. ppt

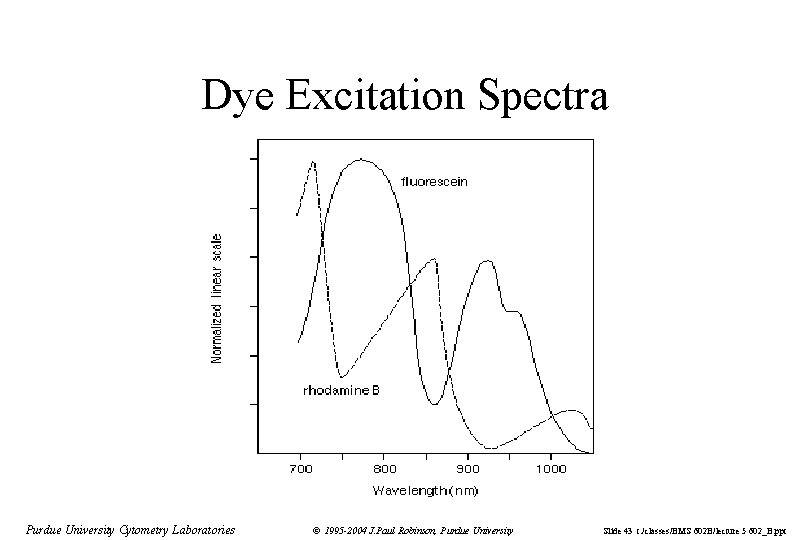
Dye Excitation Spectra Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 43 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Lasers and Probes Pulsed laser source of 1047 nm which can excite most blue and red, and some green emitting fluorophores. BLUE EMITTING: AMCA, Hoechst 33342, Hoechst 33258, DAPI GREEN EMITTING: Oregon Green 514, red-shifted GFP, JC-1, FITC, Ca Green ORANGE EMITTING: Calium Orange, Mitotracker Rosamine, Rhodamine 123, FM 4 -64 RED EMITTING: Nile Red, Calcium Crimson, TRITC, Texas Red, Di. I, PPI, CY-3 Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 44 t: /classes/BMS 602 B/lecture 5 602_B. ppt

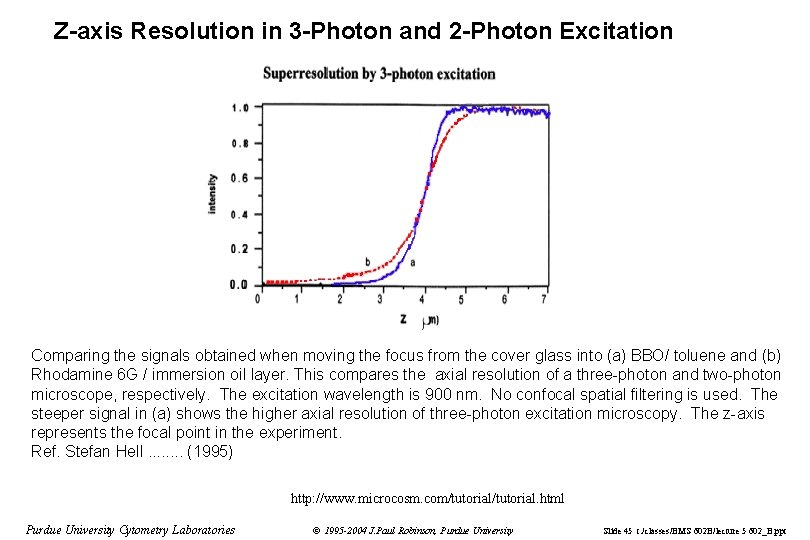
Z-axis Resolution in 3 -Photon and 2 -Photon Excitation Comparing the signals obtained when moving the focus from the cover glass into (a) BBO/ toluene and (b) Rhodamine 6 G / immersion oil layer. This compares the axial resolution of a three-photon and two-photon microscope, respectively. The excitation wavelength is 900 nm. No confocal spatial filtering is used. The steeper signal in (a) shows the higher axial resolution of three-photon excitation microscopy. The z-axis represents the focal point in the experiment. Ref. Stefan Hell. . . . (1995) http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 45 t: /classes/BMS 602 B/lecture 5 602_B. ppt

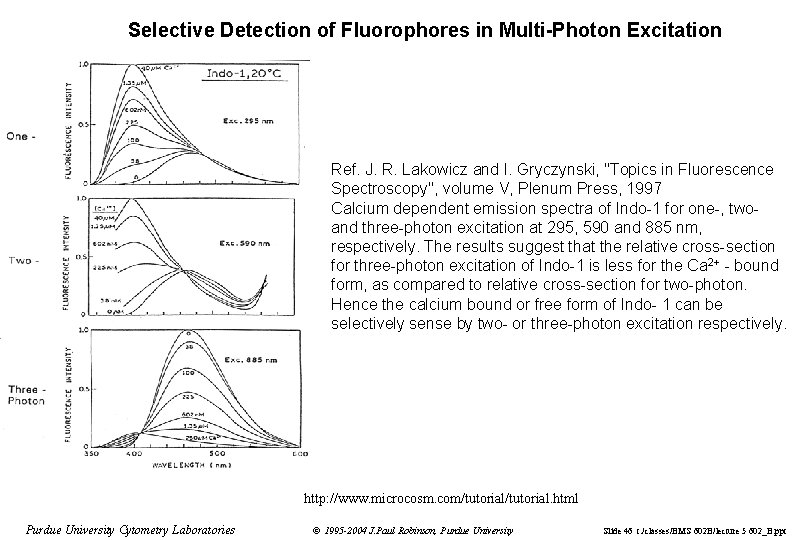
Selective Detection of Fluorophores in Multi-Photon Excitation Ref. J. R. Lakowicz and I. Gryczynski, "Topics in Fluorescence Spectroscopy", volume V, Plenum Press, 1997 Calcium dependent emission spectra of Indo-1 for one-, twoand three-photon excitation at 295, 590 and 885 nm, respectively. The results suggest that the relative cross-section for three-photon excitation of Indo-1 is less for the Ca 2+ - bound form, as compared to relative cross-section for two-photon. Hence the calcium bound or free form of Indo- 1 can be selectively sense by two- or three-photon excitation respectively. http: //www. microcosm. com/tutorial. html Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 46 t: /classes/BMS 602 B/lecture 5 602_B. ppt

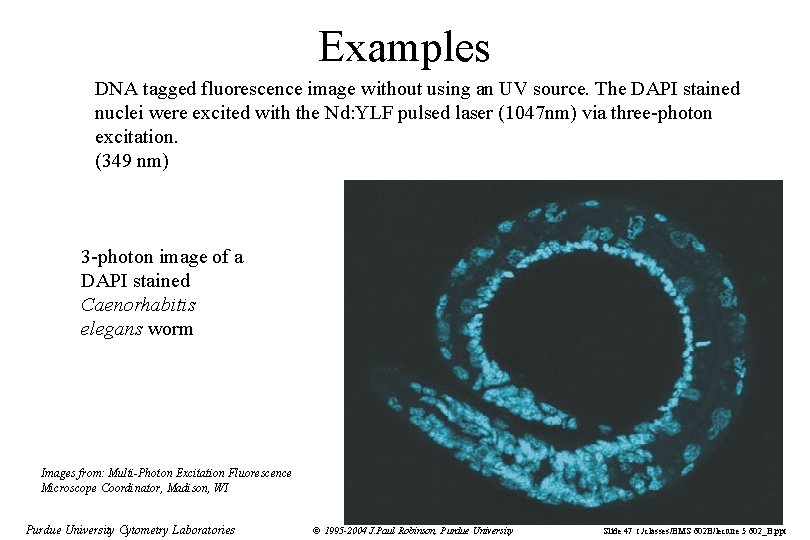
Examples DNA tagged fluorescence image without using an UV source. The DAPI stained nuclei were excited with the Nd: YLF pulsed laser (1047 nm) via three-photon excitation. (349 nm) 3 -photon image of a DAPI stained Caenorhabitis elegans worm Images from: Multi-Photon Excitation Fluorescence Microscope Coordinator, Madison, WI Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 47 t: /classes/BMS 602 B/lecture 5 602_B. ppt

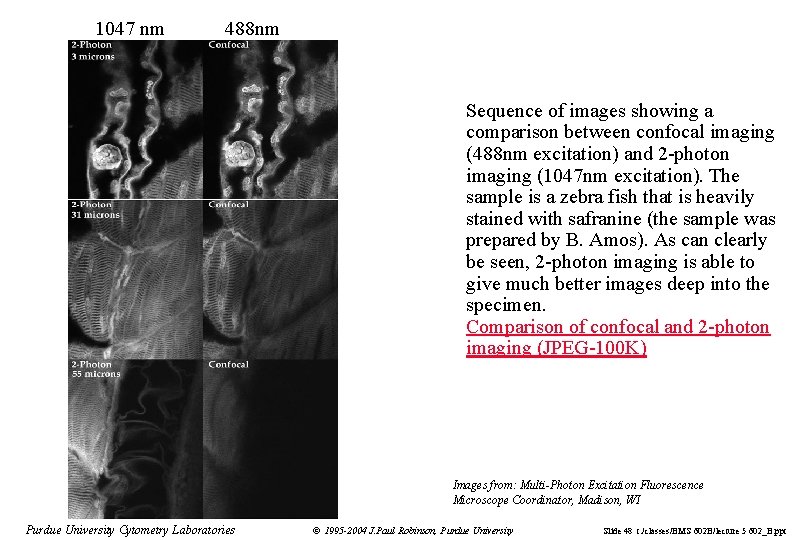
1047 nm 488 nm Sequence of images showing a comparison between confocal imaging (488 nm excitation) and 2 -photon imaging (1047 nm excitation). The sample is a zebra fish that is heavily stained with safranine (the sample was prepared by B. Amos). As can clearly be seen, 2 -photon imaging is able to give much better images deep into the specimen. Comparison of confocal and 2 -photon imaging (JPEG-100 K) Images from: Multi-Photon Excitation Fluorescence Microscope Coordinator, Madison, WI Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 48 t: /classes/BMS 602 B/lecture 5 602_B. ppt

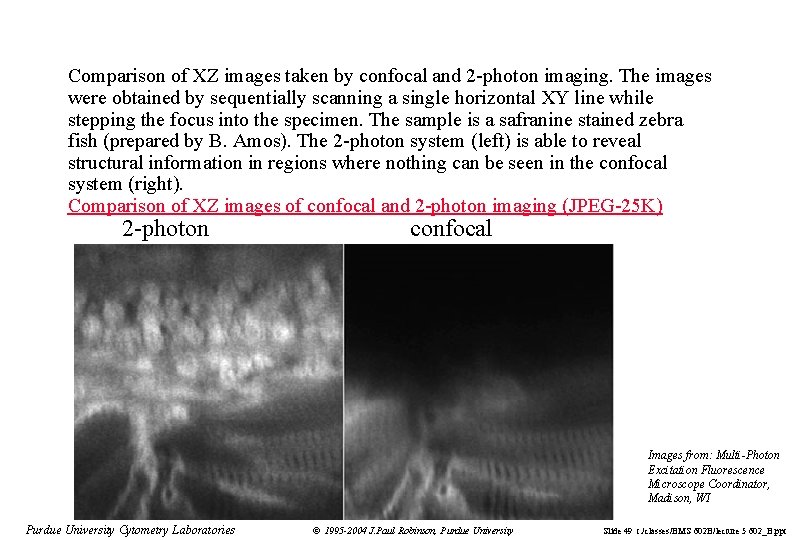
Comparison of XZ images taken by confocal and 2 -photon imaging. The images were obtained by sequentially scanning a single horizontal XY line while stepping the focus into the specimen. The sample is a safranine stained zebra fish (prepared by B. Amos). The 2 -photon system (left) is able to reveal structural information in regions where nothing can be seen in the confocal system (right). Comparison of XZ images of confocal and 2 -photon imaging (JPEG-25 K) 2 -photon confocal Images from: Multi-Photon Excitation Fluorescence Microscope Coordinator, Madison, WI Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 49 t: /classes/BMS 602 B/lecture 5 602_B. ppt

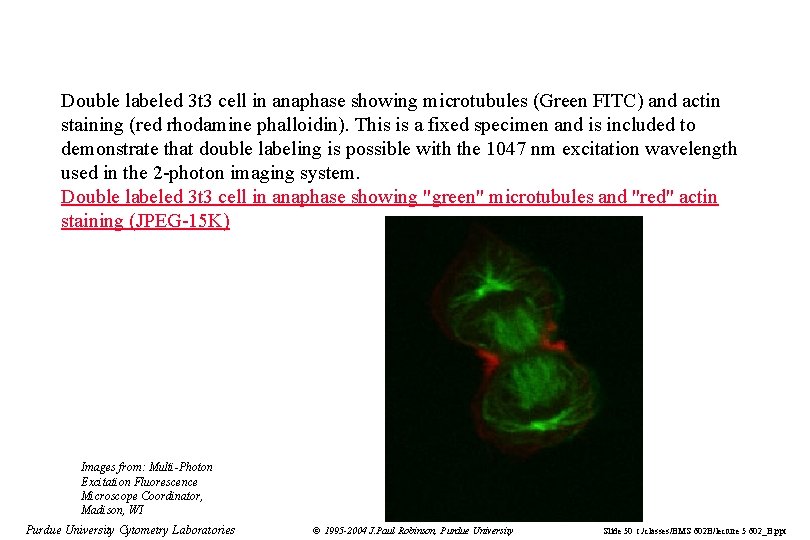
Double labeled 3 t 3 cell in anaphase showing microtubules (Green FITC) and actin staining (red rhodamine phalloidin). This is a fixed specimen and is included to demonstrate that double labeling is possible with the 1047 nm excitation wavelength used in the 2 -photon imaging system. Double labeled 3 t 3 cell in anaphase showing "green" microtubules and "red" actin staining (JPEG-15 K) Images from: Multi-Photon Excitation Fluorescence Microscope Coordinator, Madison, WI Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 50 t: /classes/BMS 602 B/lecture 5 602_B. ppt

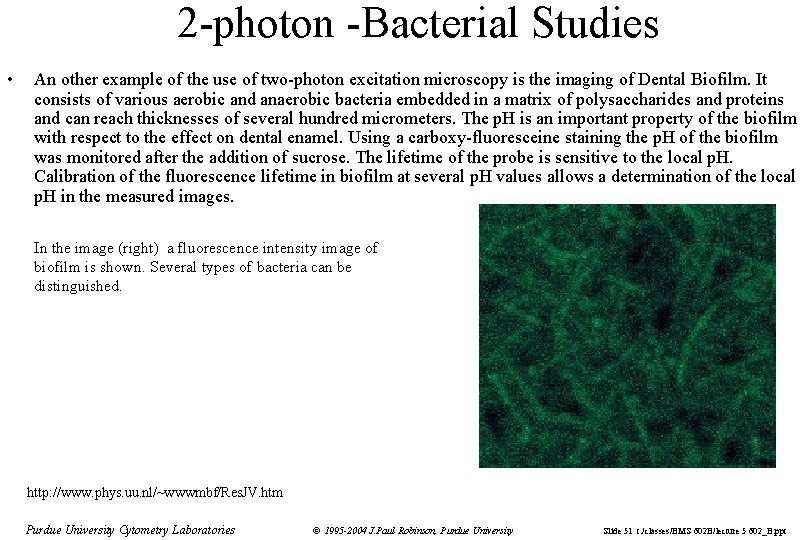
2 -photon -Bacterial Studies • An other example of the use of two-photon excitation microscopy is the imaging of Dental Biofilm. It consists of various aerobic and anaerobic bacteria embedded in a matrix of polysaccharides and proteins and can reach thicknesses of several hundred micrometers. The p. H is an important property of the biofilm with respect to the effect on dental enamel. Using a carboxy-fluoresceine staining the p. H of the biofilm was monitored after the addition of sucrose. The lifetime of the probe is sensitive to the local p. H. Calibration of the fluorescence lifetime in biofilm at several p. H values allows a determination of the local p. H in the measured images. In the image (right) a fluorescence intensity image of biofilm is shown. Several types of bacteria can be distinguished. http: //www. phys. uu. nl/~wwwmbf/Res. JV. htm Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 51 t: /classes/BMS 602 B/lecture 5 602_B. ppt

http: //www. phys. uu. nl/~wwwmbf/Res. JV. htm In the above image a fluorescence intensity image of biofilm is shown. Several types of bacteria can be distinguished. Below (left) another intensity image of biofilm is shown. After supplying the biofilm with a sucrose solution the bacterial metabolic activity increases which results in the production of H+. The fluorescence lifetime images before (middle) and 70 minutes after the addition of sucrose (right) show a clear drop in p. H. Here, the lifetime range in the images runs from p. H 6. 5 (black) to p. H 2 (white). Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 52 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Spectral Imaging • Increasing the number of spectral channels collected • Allows more advanced classification systems • Takes more time to image • Much more complex analysis Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 53 t: /classes/BMS 602 B/lecture 5 602_B. ppt

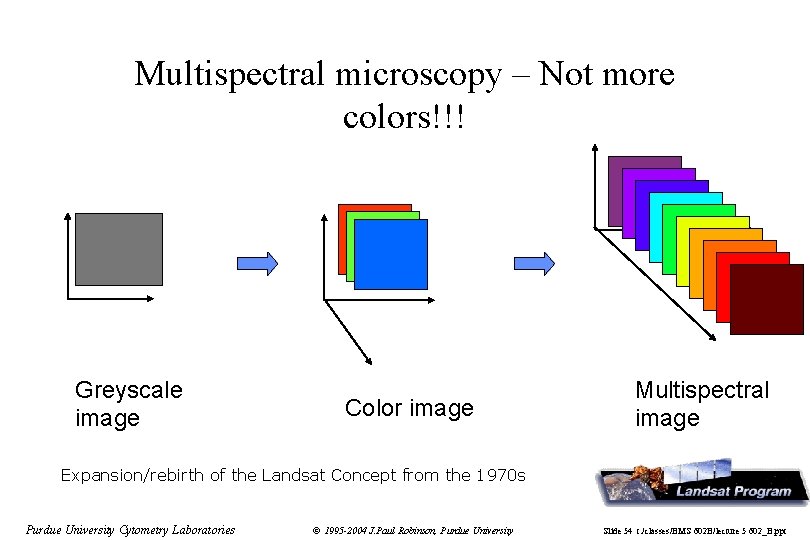
Multispectral microscopy – Not more colors!!! Greyscale image Color image Multispectral image Expansion/rebirth of the Landsat Concept from the 1970 s Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 54 t: /classes/BMS 602 B/lecture 5 602_B. ppt

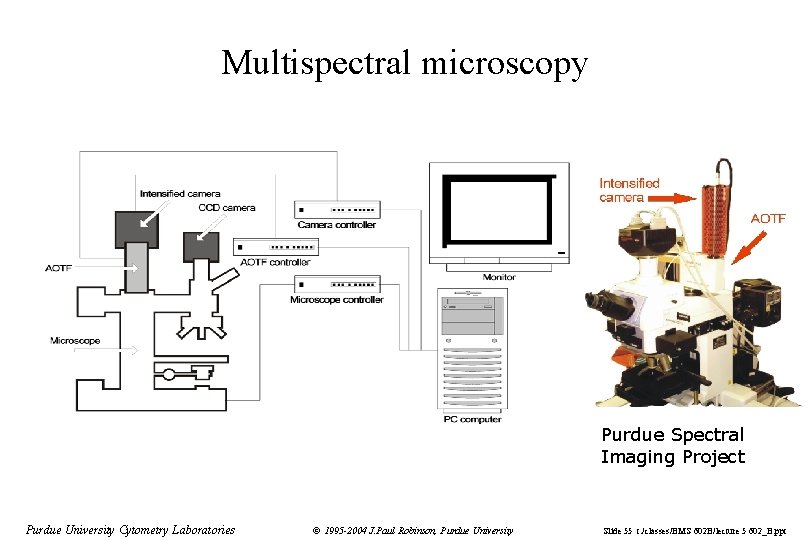
Multispectral microscopy Purdue Spectral Imaging Project Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 55 t: /classes/BMS 602 B/lecture 5 602_B. ppt

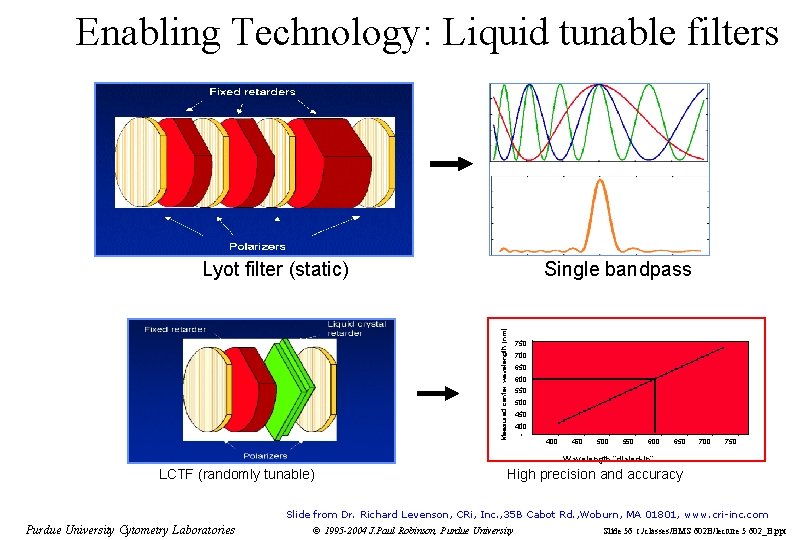
Enabling Technology: Liquid tunable filters Single bandpass Measured center wavelength (nm) Lyot filter (static) 750 700 650 600 550 500 450 400 450 500 550 600 650 700 750 Wavelength “dialed-in” LCTF (randomly tunable) High precision and accuracy Slide from Dr. Richard Levenson, CRi, Inc. , 35 B Cabot Rd. , Woburn, MA 01801, www. cri-inc. com Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 56 t: /classes/BMS 602 B/lecture 5 602_B. ppt

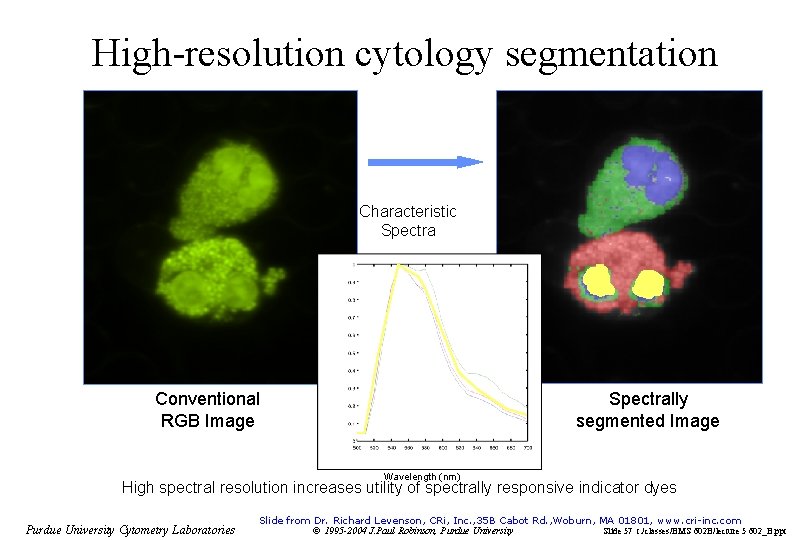
High-resolution cytology segmentation Characteristic Spectra Conventional RGB Image Spectrally segmented Image Wavelength (nm) High spectral resolution increases utility of spectrally responsive indicator dyes Purdue University Cytometry Laboratories Slide from Dr. Richard Levenson, CRi, Inc. , 35 B Cabot Rd. , Woburn, MA 01801, www. cri-inc. com © 1995 -2004 J. Paul Robinson, Purdue University Slide 57 t: /classes/BMS 602 B/lecture 5 602_B. ppt

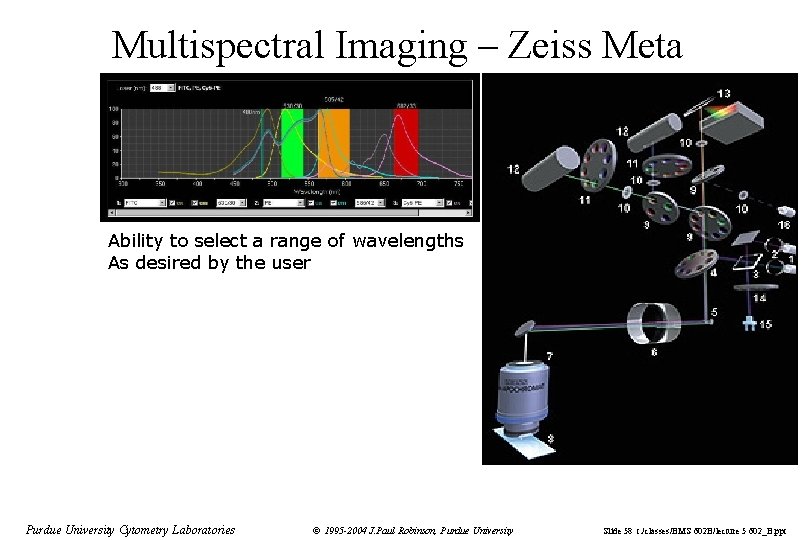
Multispectral Imaging – Zeiss Meta Ability to select a range of wavelengths As desired by the user Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 58 t: /classes/BMS 602 B/lecture 5 602_B. ppt

Nuance-Micro Slide from Dr. Richard Levenson, CRi, Inc. , 35 B Cabot Rd. , Woburn, MA 01801, www. cri-inc. com © 1995 -2004 J. Paul Robinson, Purdue University Slide 59 t: /classes/BMS 602 B/lecture 5 602_B. ppt Purdue University Cytometry Laboratories

Lecture Summary • Live cell applications are relatively common using confocal microscopy • Correct use of fluorescent probes necessary • Temperature and atmosphere control may be required • Thick specimens often require advanced image processing • Exotic applications are potentially useful • A limited window of time is available to image live cells before cells deteriorate • 2 -photon microscopy can penetrate greater tissue depth • 2 -3 photon has advantages for excitation of lower wavelengths (UV) • 2 -photon is very complex technology • 2 -photon is very expensive • Possibly the future replacing confocal imaging • Spectral Imaging will be next major change in biological imaging Purdue University Cytometry Laboratories © 1995 -2004 J. Paul Robinson, Purdue University Slide 60 t: /classes/BMS 602 B/lecture 5 602_B. ppt