Week 5 Define first ionisation energy and successive






- Slides: 26

Week 5 • Define first ionisation energy and successive ionisation energy. • Predict the number of electrons in each shell as well as the element’s group, using successive ionisation energies. • Explain the factors that influence ionisation energies. © Pearson Education Ltd 2008 This document may have been altered from the original

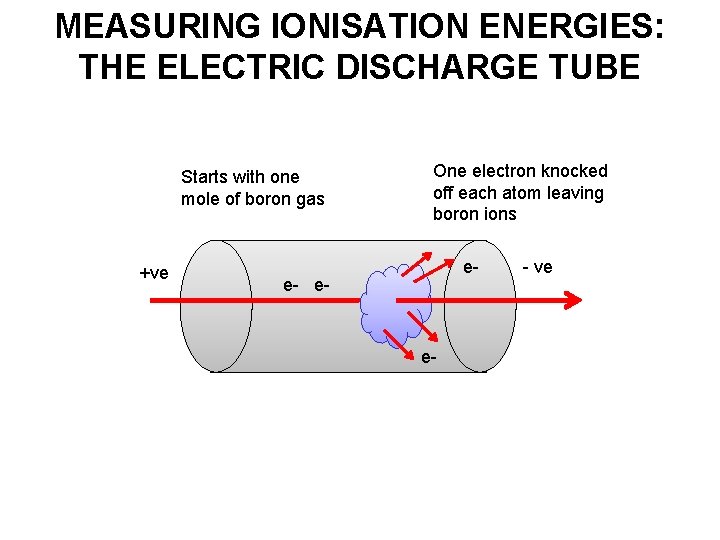
MEASURING IONISATION ENERGIES: THE ELECTRIC DISCHARGE TUBE Starts with one mole of boron gas +ve One electron knocked off each atom leaving boron ions e- e- - ve

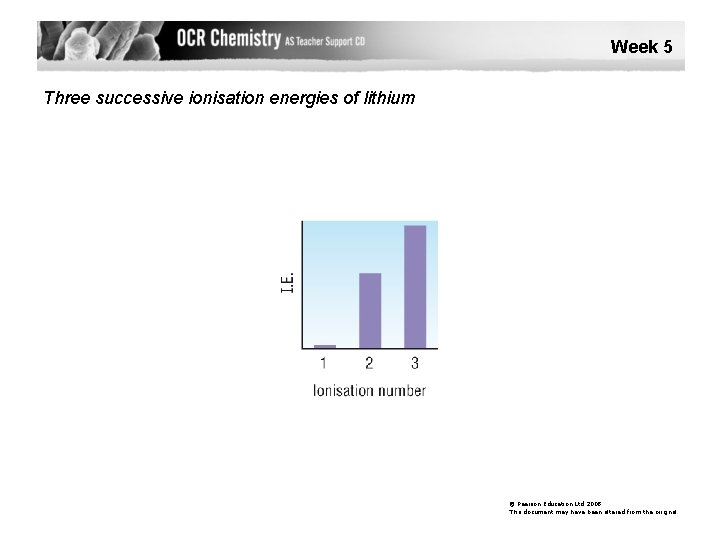
Week 5 Three successive ionisation energies of lithium © Pearson Education Ltd 2008 This document may have been altered from the original

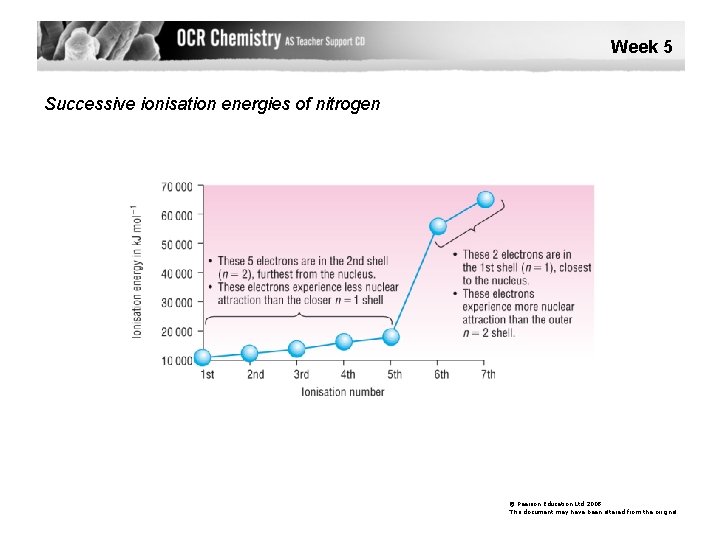
Week 5 Successive ionisation energies of nitrogen © Pearson Education Ltd 2008 This document may have been altered from the original

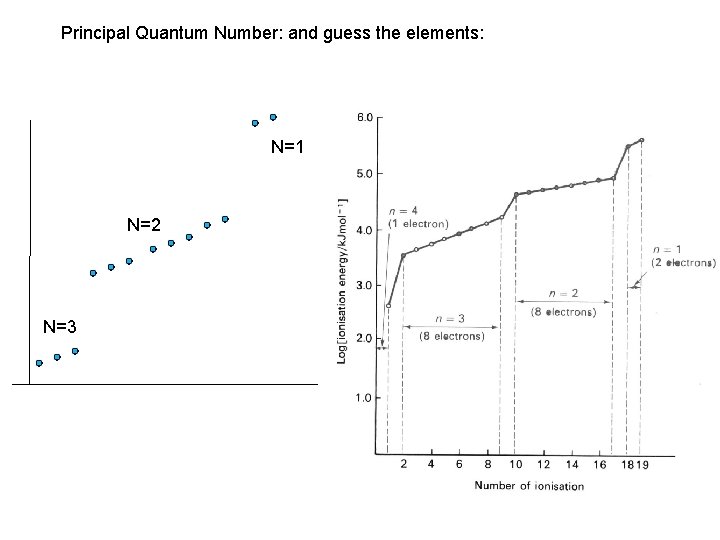
Principal Quantum Number: and guess the elements: N=1 N=2 N=3

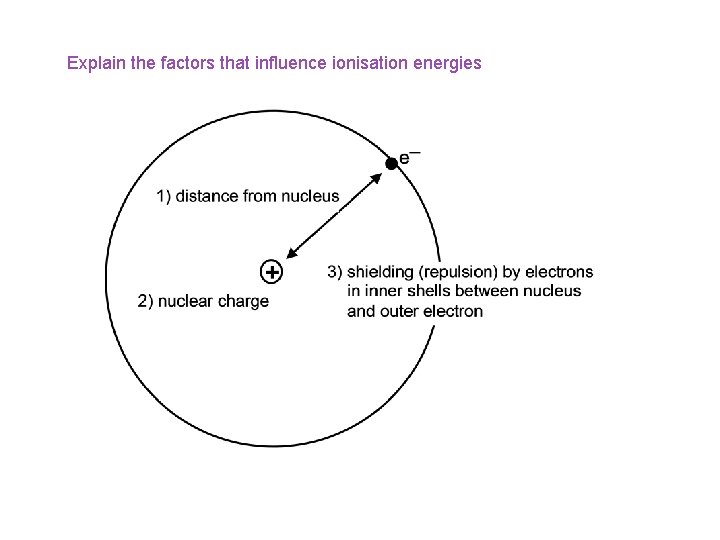
Explain the factors that influence ionisation energies

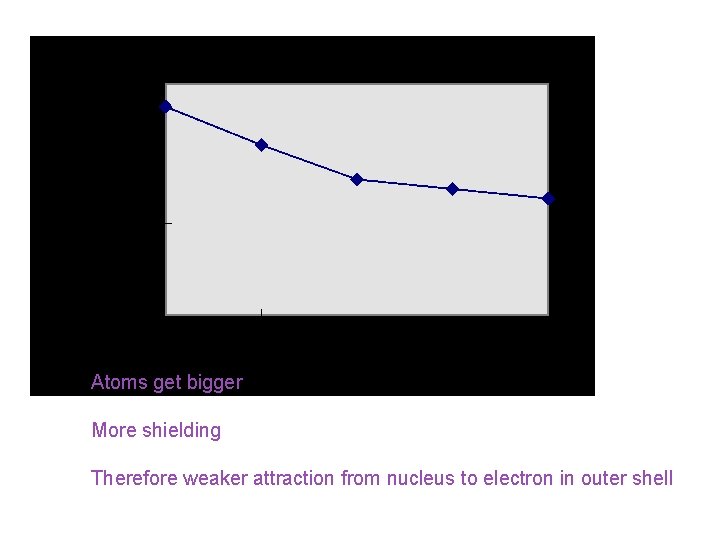
1 st ionisation energy (down group) Atoms get bigger More shielding Therefore weaker attraction from nucleus to electron in outer shell

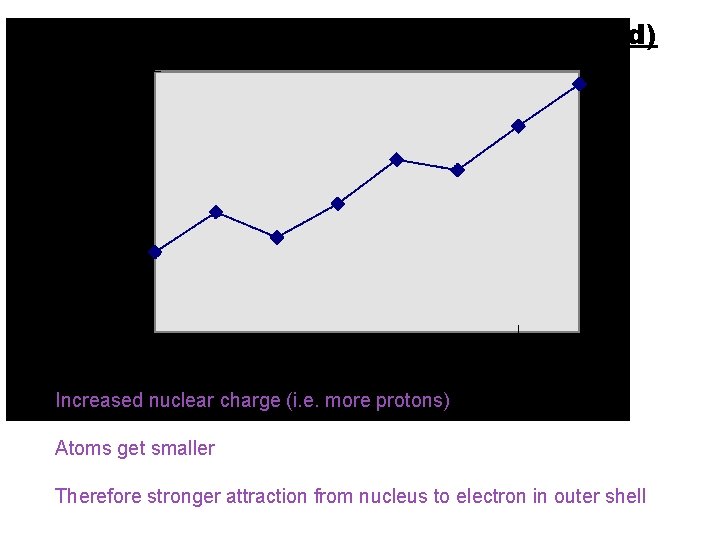
1 st ionisation energy (across period) Increased nuclear charge (i. e. more protons) Atoms get smaller Therefore stronger attraction from nucleus to electron in outer shell

Week 5 • State the number of electrons that can fill the first four shells of an atom. • Define an orbital. • Describe the shapes of s- and p-orbitals. © Pearson Education Ltd 2008 This document may have been altered from the original

Week 5 An s-orbital is spherical in shape © Pearson Education Ltd 2008 This document may have been altered from the original

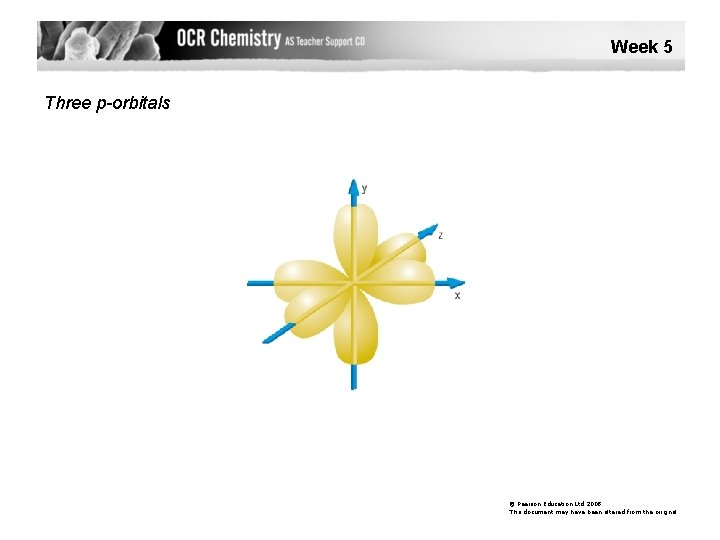
Week 5 Three p-orbitals © Pearson Education Ltd 2008 This document may have been altered from the original

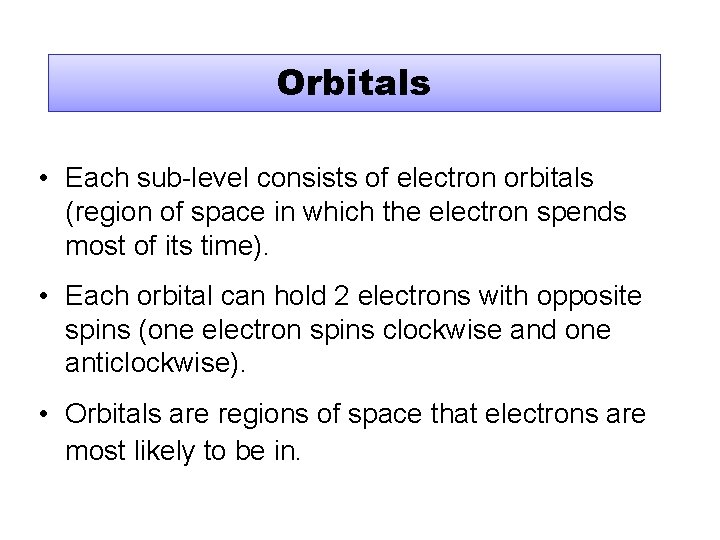
Orbitals • Each sub-level consists of electron orbitals (region of space in which the electron spends most of its time). • Each orbital can hold 2 electrons with opposite spins (one electron spins clockwise and one anticlockwise). • Orbitals are regions of space that electrons are most likely to be in.

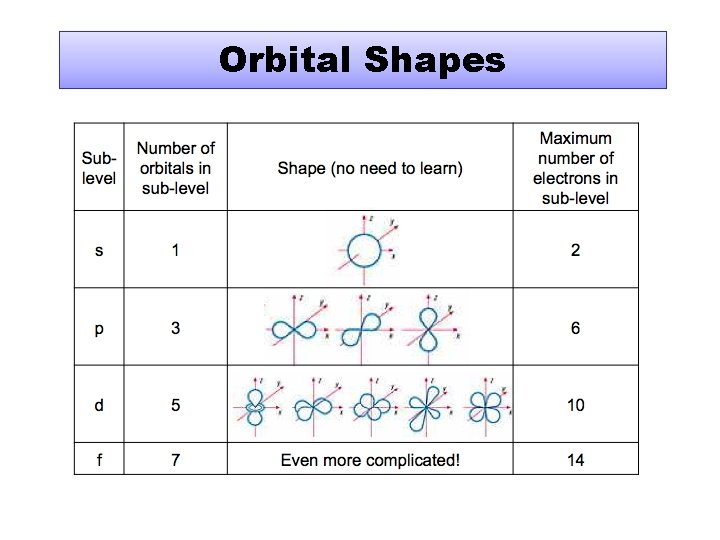
Orbital Shapes

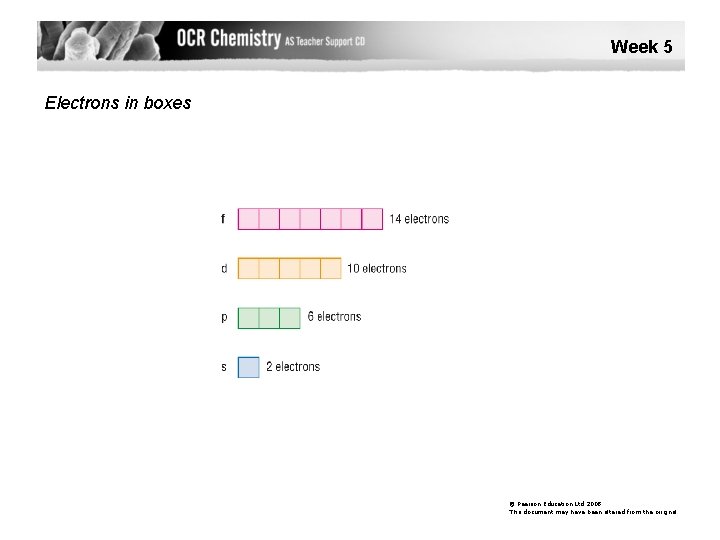
Week 5 Electrons in boxes © Pearson Education Ltd 2008 This document may have been altered from the original

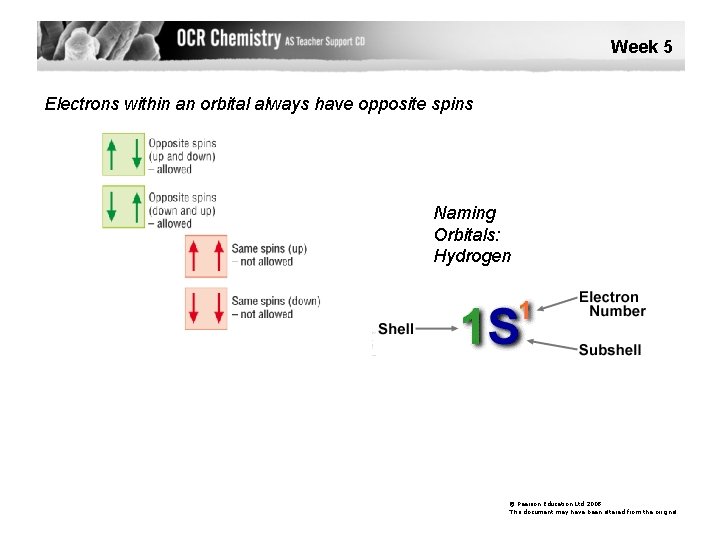
Week 5 Electrons within an orbital always have opposite spins Naming Orbitals: Hydrogen © Pearson Education Ltd 2008 This document may have been altered from the original

Week 5 • State the number of orbitals making up s, p and d sub-shells. • State the number of electrons that occupy s, p and d sub-shells. • Describe the relative energies of s-, p- and d-orbitals for shells 1, 2 and 3. • Deduce the electron configurations of atoms for the first two periods. © Pearson Education Ltd 2008 This document may have been altered from the original

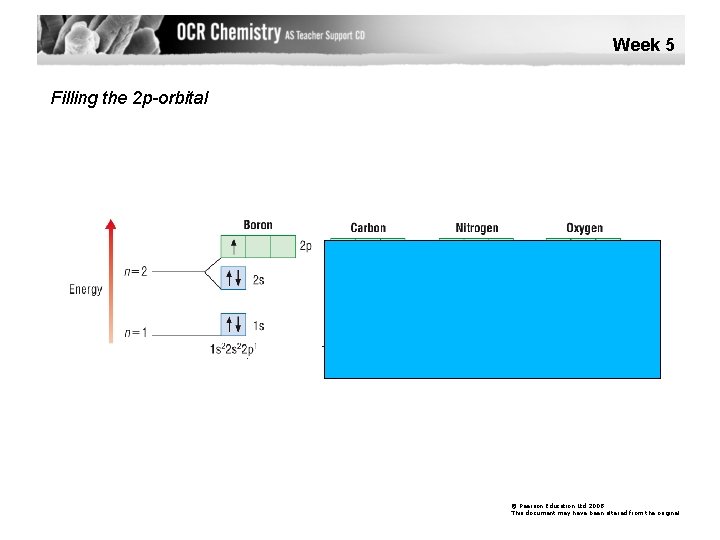
Aufbau Principle Electrons enter the lowest energy orbital available. Hund’s Rule Electrons prefer to occupy orbitals on their own, and only pair up when no empty orbitals of the same energy are available.

Week 5 Filling the 2 p-orbital © Pearson Education Ltd 2008 This document may have been altered from the original

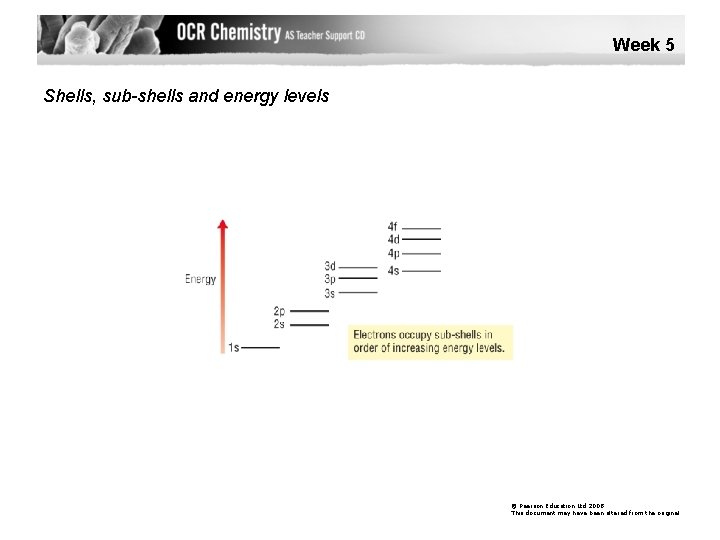
Week 5 Shells, sub-shells and energy levels © Pearson Education Ltd 2008 This document may have been altered from the original

Week 5 • Describe the relative energies of s-, p- and d-orbitals for the shells 1, 2, 3 and of the 4 s- and 4 p-orbitals. • Deduce the electron configuration of atoms and ions up to Z = 36. • Classify the elements into s-, p- and d-blocks. © Pearson Education Ltd 2008 This document may have been altered from the original

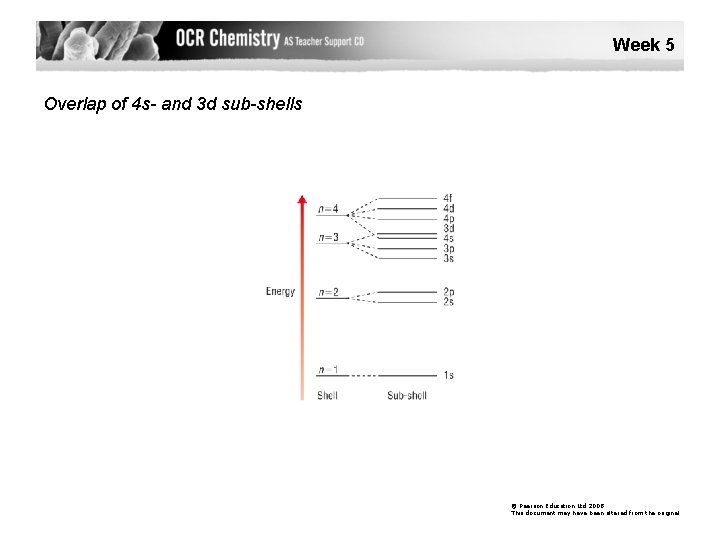
Week 5 Overlap of 4 s- and 3 d sub-shells © Pearson Education Ltd 2008 This document may have been altered from the original

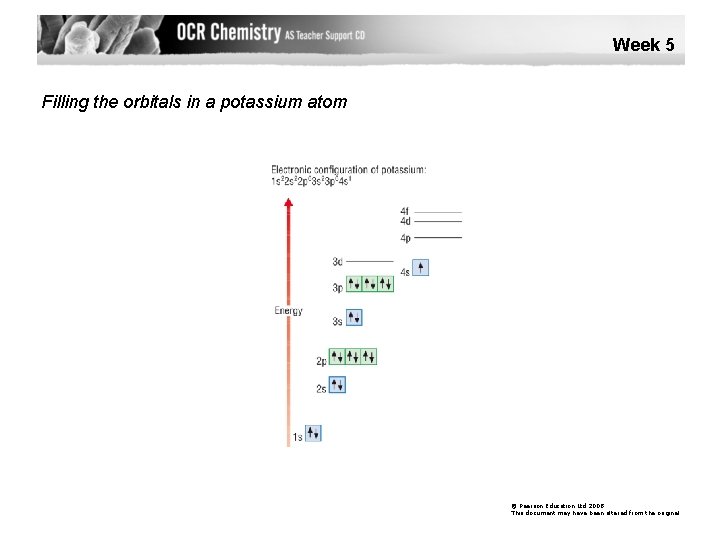
Week 5 Filling the orbitals in a potassium atom © Pearson Education Ltd 2008 This document may have been altered from the original

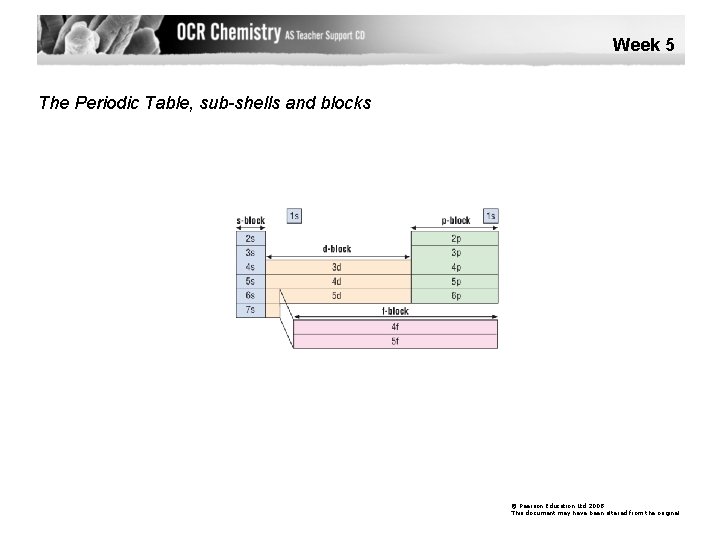
Week 5 The Periodic Table, sub-shells and blocks © Pearson Education Ltd 2008 This document may have been altered from the original

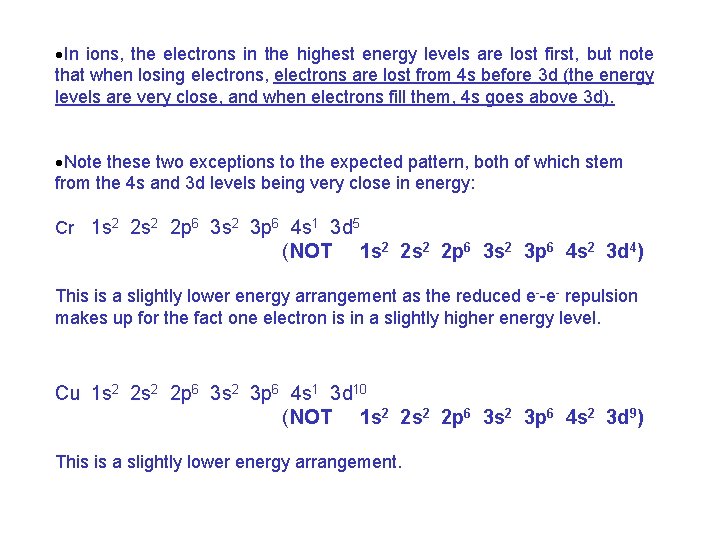
·In ions, the electrons in the highest energy levels are lost first, but note that when losing electrons, electrons are lost from 4 s before 3 d (the energy levels are very close, and when electrons fill them, 4 s goes above 3 d). ·Note these two exceptions to the expected pattern, both of which stem from the 4 s and 3 d levels being very close in energy: Cr 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 (NOT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 4) This is a slightly lower energy arrangement as the reduced e--e- repulsion makes up for the fact one electron is in a slightly higher energy level. Cu 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 (NOT 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 9) This is a slightly lower energy arrangement.

