Wednesday 18 th November Naming Compounds LO Learn
Wednesday 18 th November Naming Compounds LO: Learn how to name compounds in chemical reactions. Key Words: Compound Equation Learning challenge Interpret word and formula equations to determine the name of the compound. Extended challenge Balance formula equations. LIFELINES: READING: R 4 TALKING: T 2 WRITING: W 5
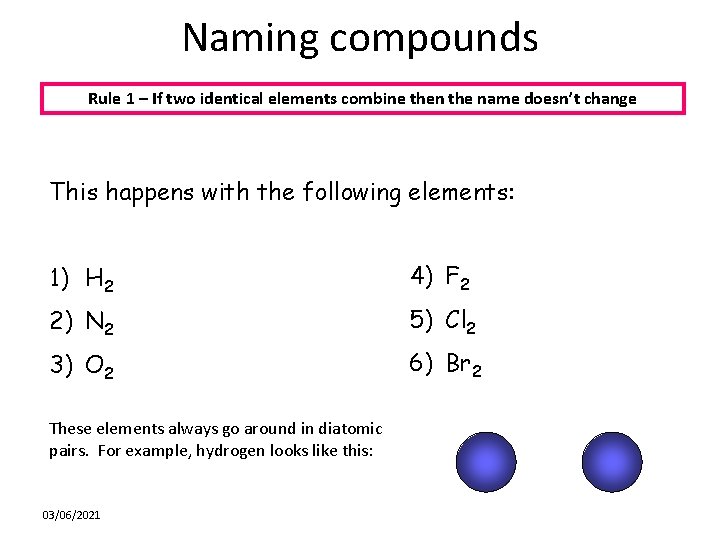
Naming compounds Rule 1 – If two identical elements combine then the name doesn’t change This happens with the following elements: 1) H 2 4) F 2 2) N 2 5) Cl 2 3) O 2 6) Br 2 These elements always go around in diatomic pairs. For example, hydrogen looks like this: 03/06/2021
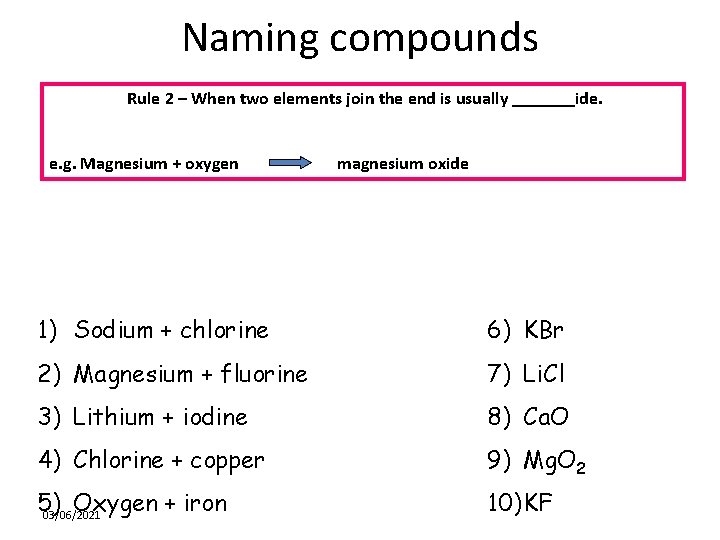
Naming compounds Rule 2 – When two elements join the end is usually _______ide. e. g. Magnesium + oxygen magnesium oxide 1) Sodium + chlorine 6) KBr 2) Magnesium + fluorine 7) Li. Cl 3) Lithium + iodine 8) Ca. O 4) Chlorine + copper 9) Mg. O 2 5) Oxygen + iron 03/06/2021 10) KF
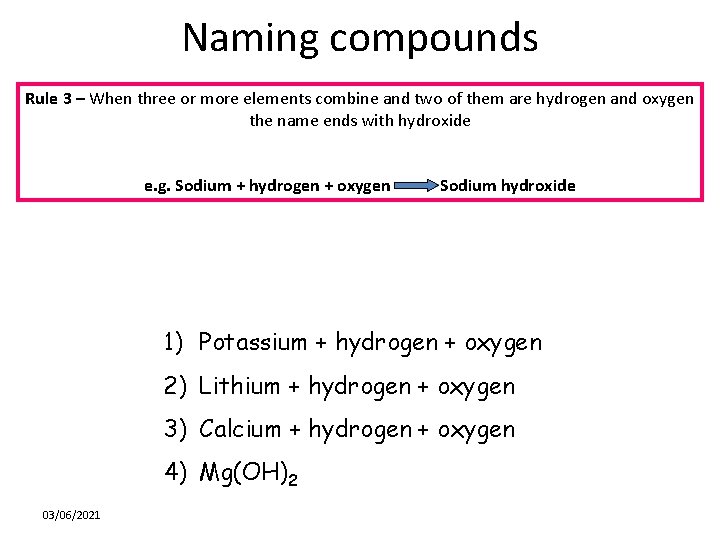
Naming compounds Rule 3 – When three or more elements combine and two of them are hydrogen and oxygen the name ends with hydroxide e. g. Sodium + hydrogen + oxygen Sodium hydroxide 1) Potassium + hydrogen + oxygen 2) Lithium + hydrogen + oxygen 3) Calcium + hydrogen + oxygen 4) Mg(OH)2 03/06/2021
Naming compounds Rule 4 – When three or more elements combine and one of them is oxygen the ending is _____ate e. g. Copper + sulphur + oxygen Copper sulphate 1) Calcium + carbon + oxygen 6) Ag. NO 3 2) Potassium + carbon + oxygen 7) H 2 SO 4 3) Calcium + sulphur + oxygen 8) K 2 CO 3 4) Magnesium + chlorine + oxygen 5) Calcium + oxygen + nitrogen 03/06/2021
- Slides: 5