WedgeDash Notation QUEST Organic Chemistry Depicting 3 D





- Slides: 12

Wedge-Dash Notation QUEST Organic Chemistry

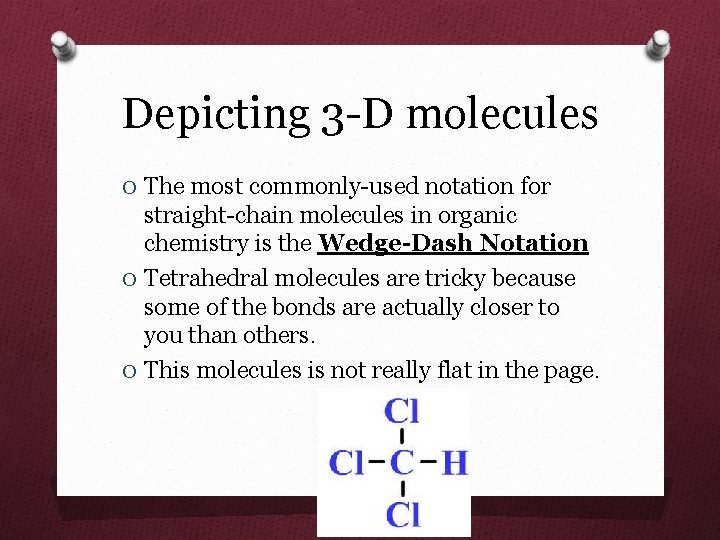
Depicting 3 -D molecules O The most commonly-used notation for straight-chain molecules in organic chemistry is the Wedge-Dash Notation O Tetrahedral molecules are tricky because some of the bonds are actually closer to you than others. O This molecules is not really flat in the page.

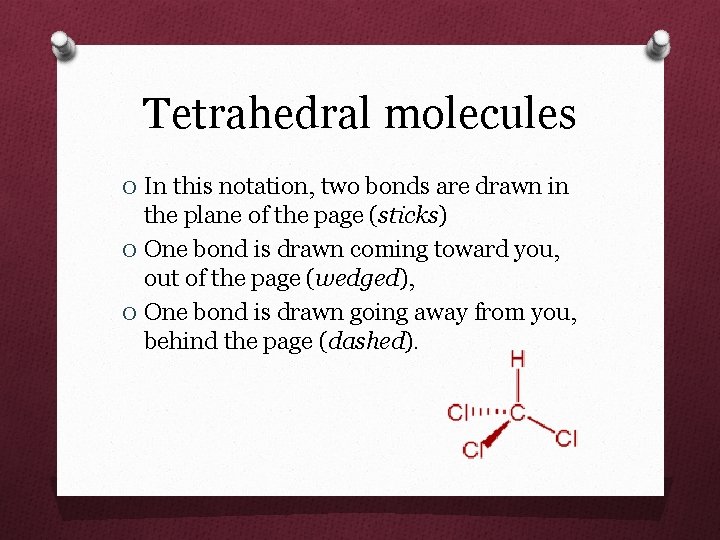
Tetrahedral molecules O In this notation, two bonds are drawn in the plane of the page (sticks) O One bond is drawn coming toward you, out of the page (wedged), O One bond is drawn going away from you, behind the page (dashed).

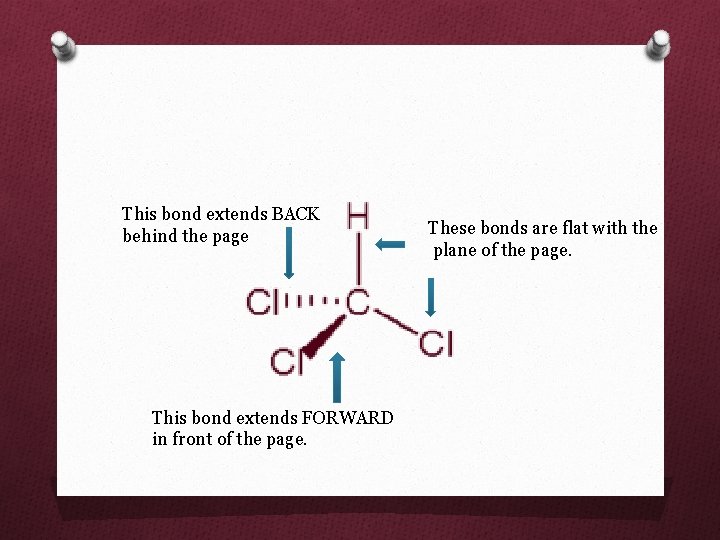
This bond extends BACK behind the page This bond extends FORWARD in front of the page. These bonds are flat with the plane of the page.

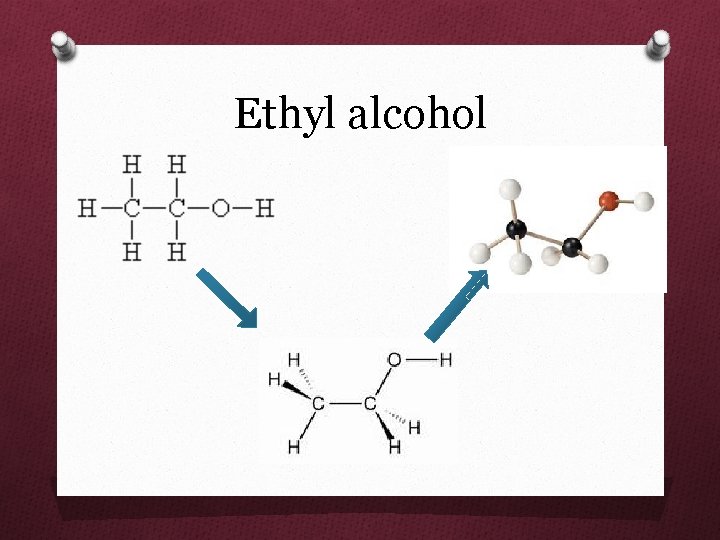
Ethyl alcohol

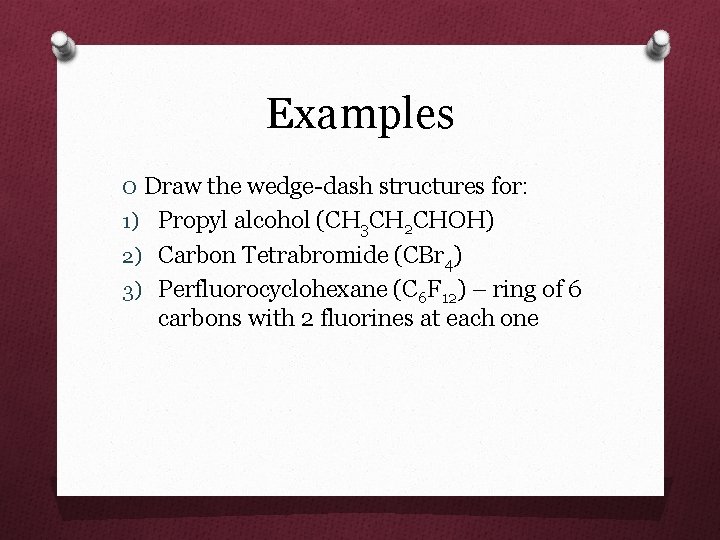
Examples O Draw the wedge-dash structures for: 1) Propyl alcohol (CH 3 CH 2 CHOH) 2) Carbon Tetrabromide (CBr 4) 3) Perfluorocyclohexane (C 6 F 12) – ring of 6 carbons with 2 fluorines at each one

Write the formula for this molecule O Remember that the ends of lines represent carbons and have an appropriate # of hydrogens attached

Quiz O On the labeled carbon atom, what type of stick goes to the hydrogen atom (not drawn)? What direction is this?

Quiz O In the structure above, the alcohol on the second carbon is behind the page. Since there must be four bonds to each carbon, and two sticks and one dash have been drawn in, the remaining hydrogen must be wedged. O It is coming out of the page toward you!

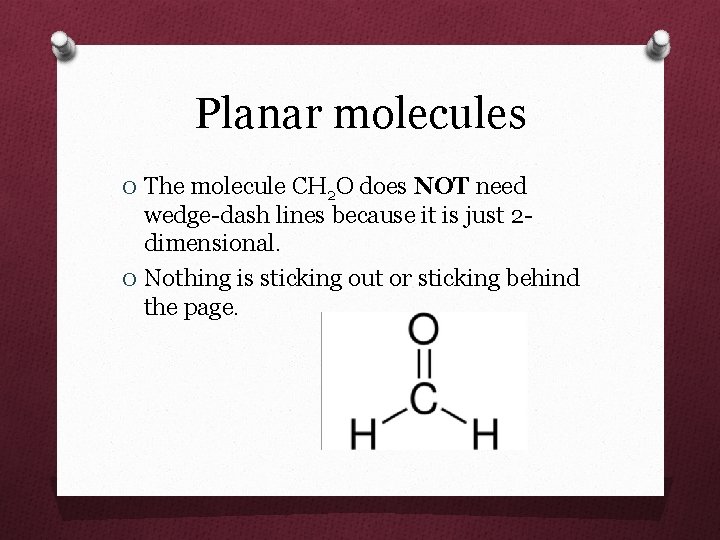
Planar molecules O The molecule CH 2 O does NOT need wedge-dash lines because it is just 2 dimensional. O Nothing is sticking out or sticking behind the page.

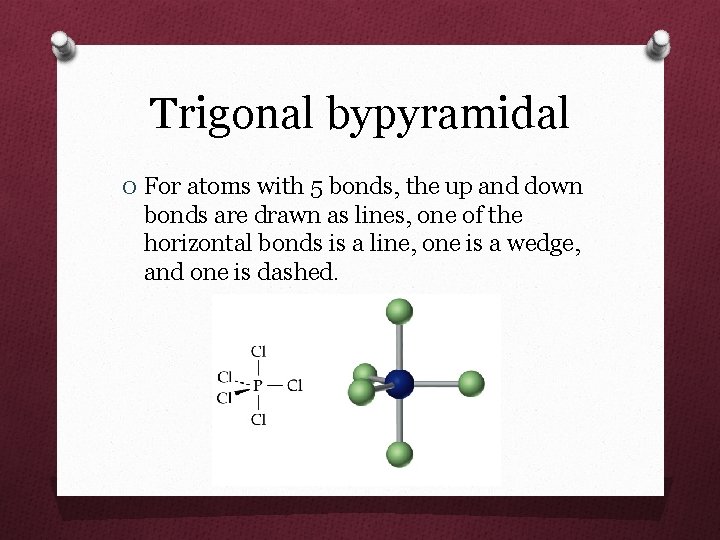
Trigonal bypyramidal O For atoms with 5 bonds, the up and down bonds are drawn as lines, one of the horizontal bonds is a line, one is a wedge, and one is dashed.

Quiz O Try the wedge-dash structure for PCl 3 Br 2 O (Hint: draw PCl 5 then substitute 2 bromines)