WEATHERING Prepared by Rana Faizan Saleem BSApplied Geology


WEATHERING Prepared by: Rana Faizan Saleem BS-Applied Geology Institute of Geology, Universty of The Punjab

AIM OF THIS PRESENTATION Is to explain you about: Ø What is Weathering? Ø Agents of Weathering Ø Types of Mechanical Weathering Ø Types of Chemical Weathering Ø Factors that Effects rate of Weathering

WEATHERING Ø Weathering is the Physical and Chemical breakdown of pre-existing rocks into small particles , called sediments.

AGENTS OF WEATHERING Water Ø Wind Ø Ice Ø Gravity Ø

TYPES OF WEATHERING There are following types of Weathering: Ø Mechanical Weathering Ø Chemical Weathering

MECHANICAL WEATHERING Ø The Physical breakdown of rocks into sediments, but their Chemical composition does not change.

TYPES OF MECHANICAL WEATHERING Frost Wedging Ø Salt Wedging Ø Exfoliation Ø Frost Heaving Ø Organic Activity Ø Abrasion Ø

FROST WEDGING When water peels into the cracks of rocks, it freezes and causes the rock to crack.
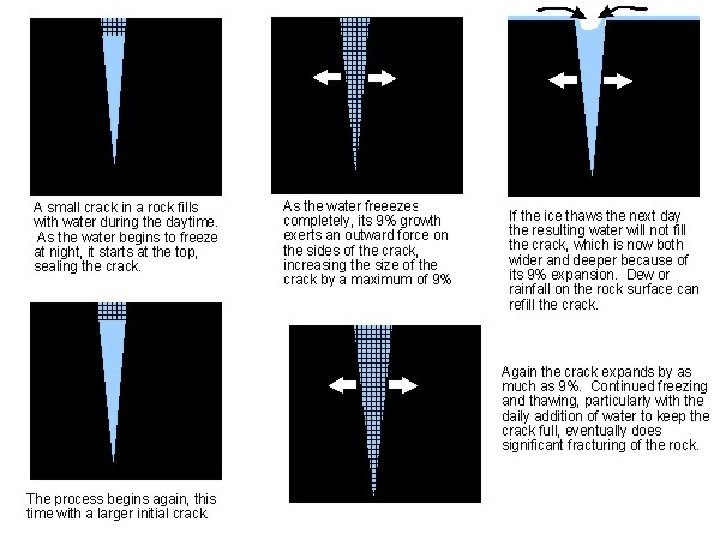


SALT WEDGING Ø This occurs when rain water fills cracks in rocks; then water evaporate and with the passage of time salt crystals grow into cracks and expands, finally break the rocks.



EXFOLIATION When a rock has heat exerted upon it, along with pressure, it separates into layers. It is also called Onion Skin Weathering because rock layers separate like onion layers.



FROST HEAVING Upward or Vertical movement of soil due to freezing of water in soil.

ORGANIC ACTIVITY Ø Organic weathering is the breakdown of rocks by plants or animal action or by chemicals formed from plants and animals.


ABRASION Abrasion occurs when rocks rub against each other and become rounded.


CHEMICAL WEATHERING Ø The process by which rocks break down into sediments and their chemical composition will also changed.

TYPES OF CHEMICAL WEATHERING Hydrolysis Ø Oxidation Ø Carbonation Ø Hydration Ø Reduction Ø Acid Rain Ø Plant Acid Ø

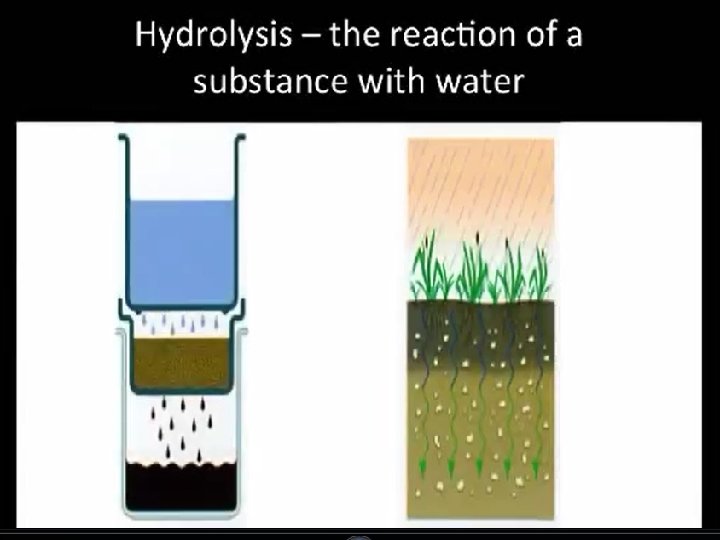
HYDROLYSIS When rocks sit in water for extended periods of time they begin to break down and have a clay-like texture. 2 KAl. Si 3 O 8+ 2 H+ + H 2 O → Al 2 Si 2 O 5(OH)4 +2 K +4 Si. O 2 (Orthoclase) (Kaolinite)
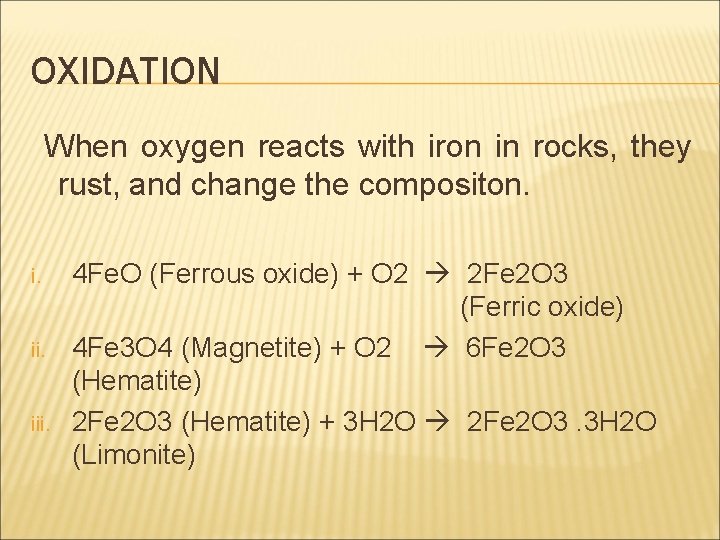
OXIDATION When oxygen reacts with iron in rocks, they rust, and change the compositon. i. iii. 4 Fe. O (Ferrous oxide) + O 2 2 Fe 2 O 3 (Ferric oxide) 4 Fe 3 O 4 (Magnetite) + O 2 6 Fe 2 O 3 (Hematite) 2 Fe 2 O 3 (Hematite) + 3 H 2 O 2 Fe 2 O 3. 3 H 2 O (Limonite)


CARBONATION Ø i. ii. The carbon dioxide dissolved in water forms carbonic acid that reacts with many common minerals. e. g Limestone and Calcite. CO 2 + H 2 O H 2 CO 3 carbonic acid H 2 CO 3 + Ca. CO 3 Ca++ + 2 HCO 3 - (bicarbonate ions)
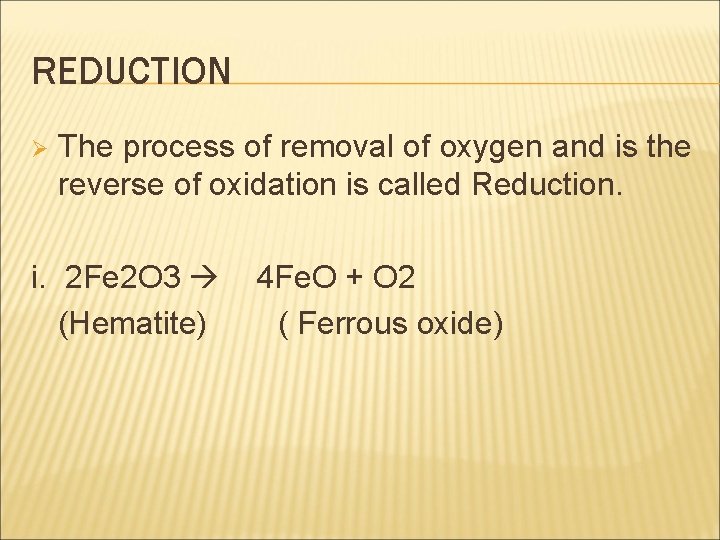
REDUCTION Ø The process of removal of oxygen and is the reverse of oxidation is called Reduction. i. 2 Fe 2 O 3 4 Fe. O + O 2 (Hematite) ( Ferrous oxide)

HYDRATION Ø i. ii. Chemical combination of water molecules with a particular substance or mineral leading to a change in structure. Al 2 O 3 + 3 HOH Al 2 O 3. 3 H 2 O (Bauxite) (Hyd. aluminium Oxide) Ca. SO 4 + 2 H 2 O Ca. SO 4. 2 H 2 O (Anhydrite) (Gypsum)

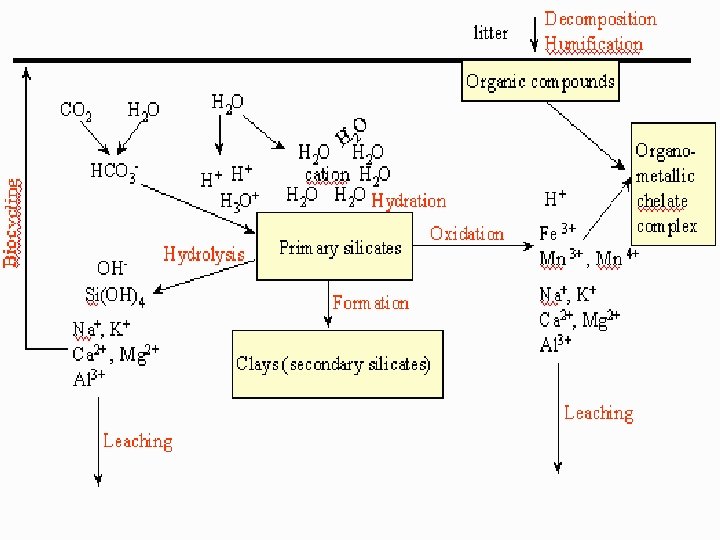

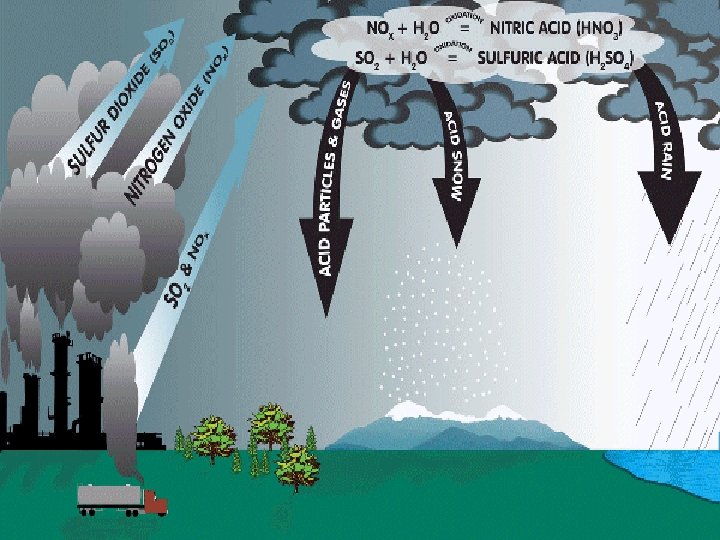
ACID RAIN Ø Water in the atmosphere absorbs sulfur oxides and nitrogen oxides and forms Sulfuric Acid and Nitric Acid which falls in the form of rain on earth called Acid Rain. Ø It changs the composition of minerals and rocks.



PLANT ACID Ø Plant Acid- When plants decay they release acids that react with the minerals in rocks.


FACTORS THAT AFFECTING RATE OF WEATHERING Ø Climate Ø Rock Composition Ø Surface Area

CLIMATE Ø Weathering occurs fastest in hot, wet climates. It occurs very slowly in hot, dry climates. Without temperature changes, ice wedging cannot occur. In very cold, dry areas, there is little weathering.

ROCK COMPOSITION Ø Some minerals resist weathering. Quartz is a mineral that weathers slowly. Rocks made up of minerals such as feldspar, calcite, and iron, weather more quickly.

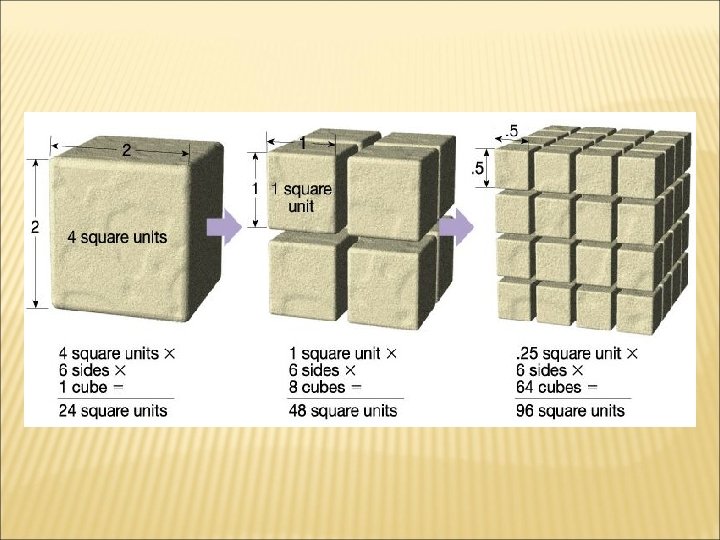
SURFACE AREA Ø The more surface area a rock has, the more quickly it will weather. Ø When a block is cut into smaller pieces, it has more surface area. So, therefore, the smaller pieces of a rock will weather faster than a large block of rock

THANK YOU FOR YOUR ATTENTION
- Slides: 45