Weak Acids Ka p Ka Polyprotic Acids WARNING

![What is the p. H of a solution with [OH-] = 3. 58 x What is the p. H of a solution with [OH-] = 3. 58 x](https://slidetodoc.com/presentation_image/4f0791ed9f383eebd2df77ac06af0f48/image-5.jpg)


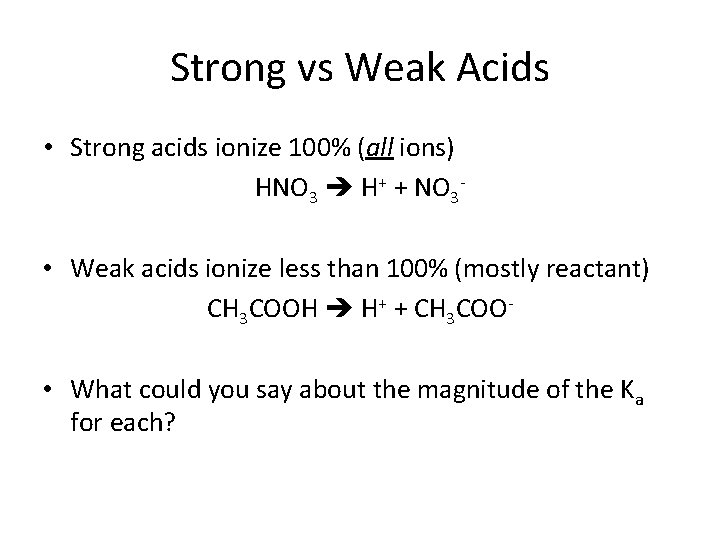






![Determine the [H+], the p. H and the [PO 43 -] in an aqueous Determine the [H+], the p. H and the [PO 43 -] in an aqueous](https://slidetodoc.com/presentation_image/4f0791ed9f383eebd2df77ac06af0f48/image-22.jpg)
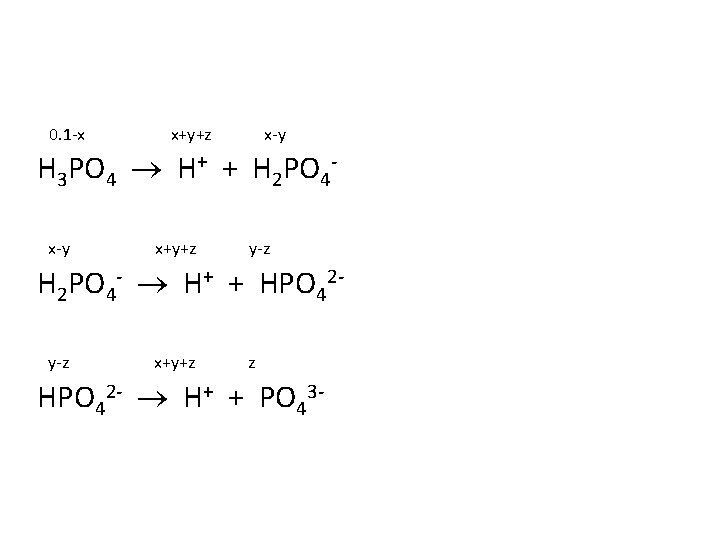
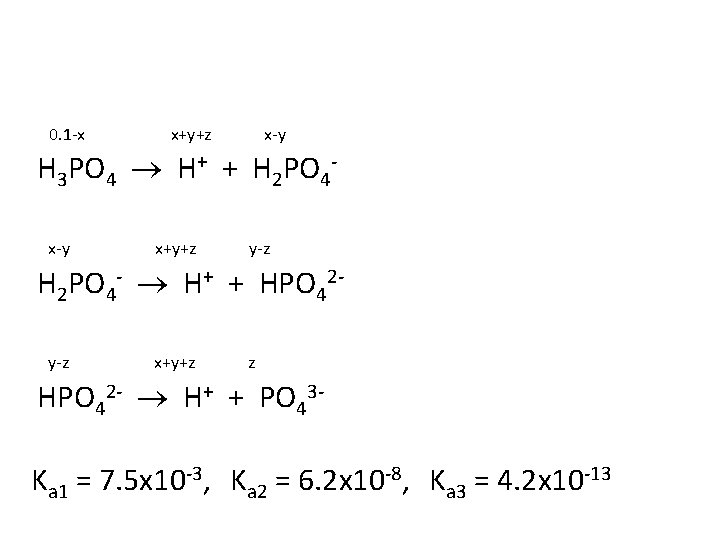
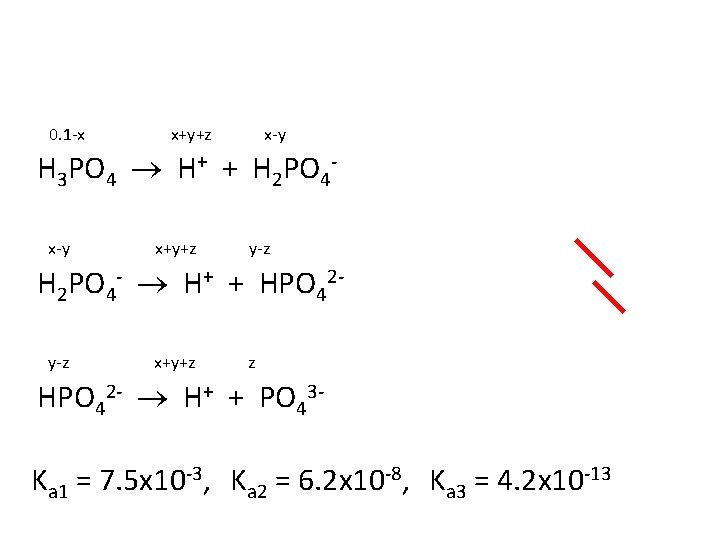
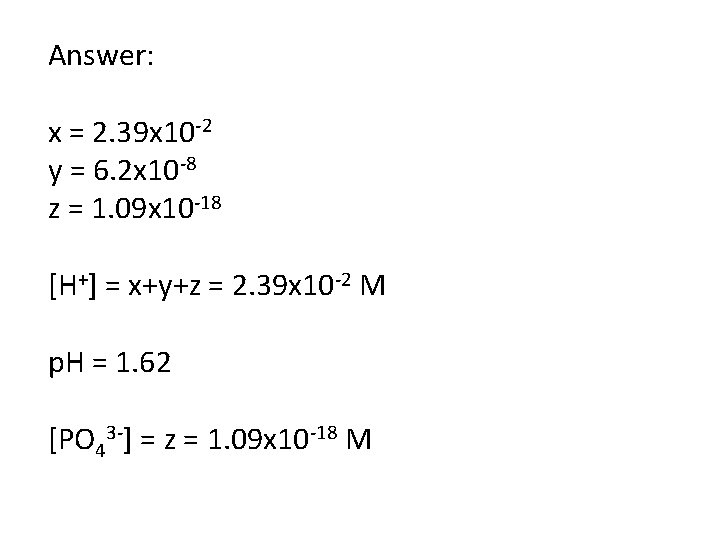
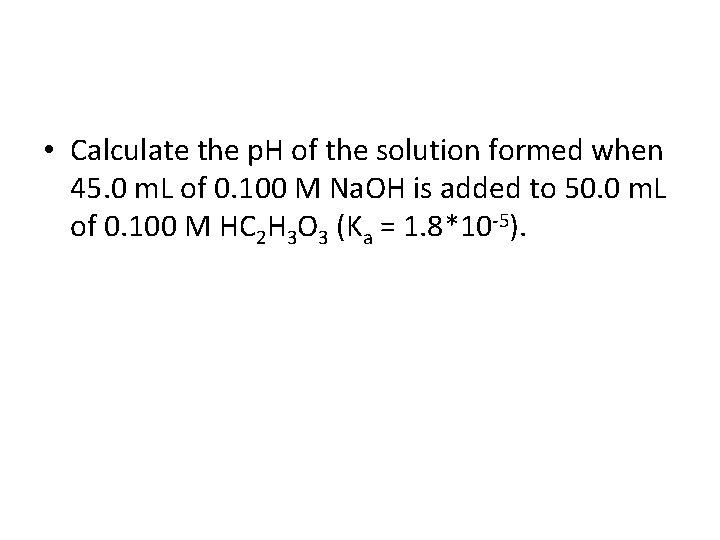
- Slides: 28

Weak Acids, Ka, p. Ka Polyprotic Acids

WARNING: You will not do well next week unless you understand this week very well! You must work hard on this chapter in order to understand titrations next week.

Determine the acid (A), base (B), conjugate acid (CA), conjugate base (CB) for the reaction: CN- + HCl. O HCN + Cl. O- A. B. C. D. A, B, CA, CB CB, CA, A, B B, A, CB CA, CB, B, A

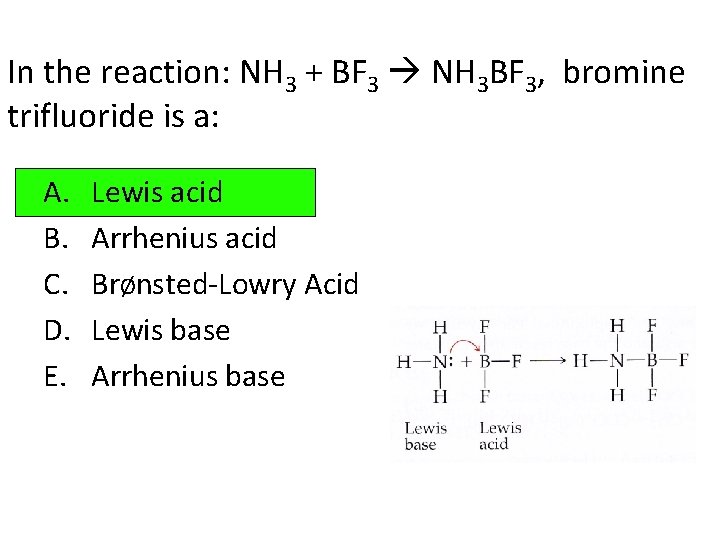
In the reaction: NH 3 + BF 3 NH 3 BF 3, bromine trifluoride is a: A. B. C. D. E. Lewis acid Arrhenius acid BrØnsted-Lowry Acid Lewis base Arrhenius base
![What is the p H of a solution with OH 3 58 x What is the p. H of a solution with [OH-] = 3. 58 x](https://slidetodoc.com/presentation_image/4f0791ed9f383eebd2df77ac06af0f48/image-5.jpg)
What is the p. H of a solution with [OH-] = 3. 58 x 10 -9 M? A. B. C. D. 8. 45 8. 27 7. 99 5. 55

Find the p. H of a solution prepared with 45 g Sr(OH)2 in 600 m. L. (MW = 122 g/mol) A. B. C. D. 0. 0899 14. 1 0. 211 13. 8

HNO 2 is a strong acid A. True B. False

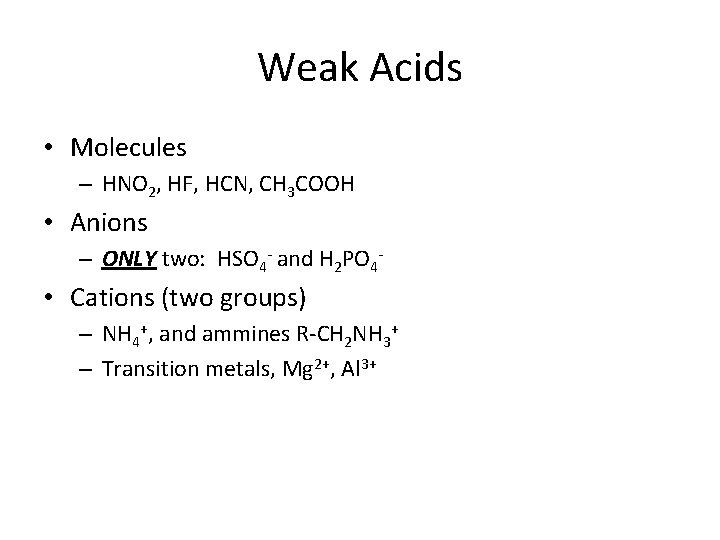
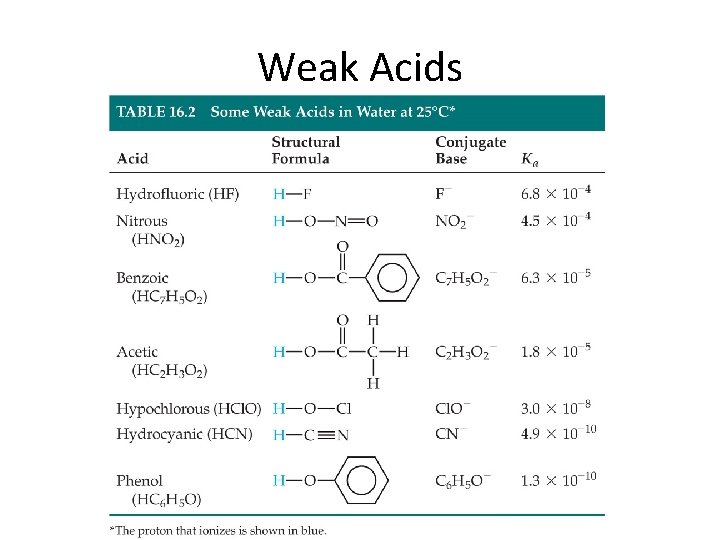
Weak Acids • Molecules – HNO 2, HF, HCN, CH 3 COOH • Anions – ONLY two: HSO 4 - and H 2 PO 4 - • Cations (two groups) – NH 4+, and ammines R-CH 2 NH 3+ – Transition metals, Mg 2+, Al 3+
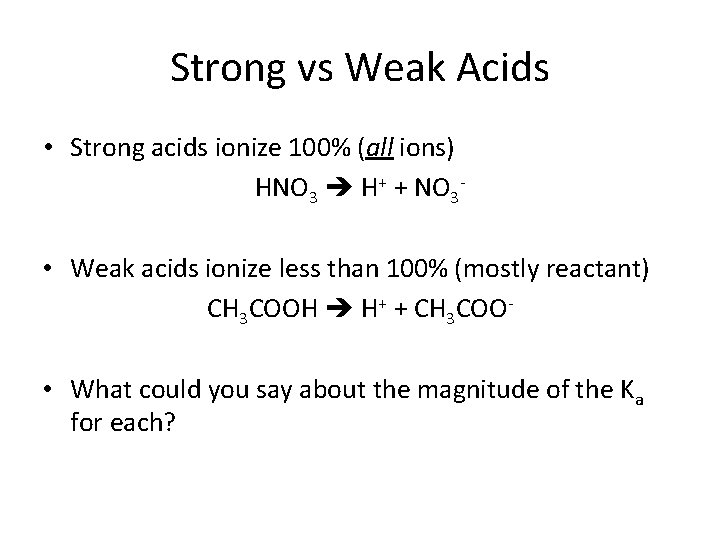
Strong vs Weak Acids • Strong acids ionize 100% (all ions) HNO 3 H+ + NO 3 • Weak acids ionize less than 100% (mostly reactant) CH 3 COOH H+ + CH 3 COO • What could you say about the magnitude of the Ka for each?

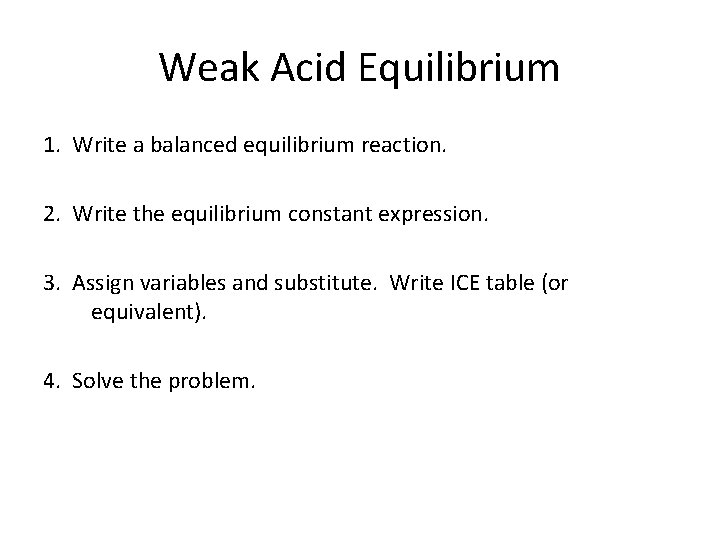
Weak Acid Equilibrium 1. Write a balanced equilibrium reaction. 2. Write the equilibrium constant expression. 3. Assign variables and substitute. Write ICE table (or equivalent). 4. Solve the problem. Does this sound familiar?

Caproic acid, HC 6 H 11 O 2, is found in coconut oil and is used in making artificial flavors. A solution is made by dissolving 0. 450 mol of caproic acid in enough water to make 2. 0 L of solution. The solution has [H+] = 1. 7 x 10 -3 M. What is the Ka for caproic acid? Answer: 1. 29 x 10 -5 What would you do if the same question gave you a p. H instead of [H+]?

Butyric acid, HC 4 H 7 O 2, is responsible for the odor of rancid butter and cheese. Its Ka is 1. 51 x 10 -5. Calculate the p. H when 13. 5 g are dissolved in enough water to make 1. 30 L. (MW=88 g/mol) Answer: 2. 87

Weak Acid Equilibrium 1. Write a balanced equilibrium reaction. 2. Write the equilibrium constant expression. 3. Assign variables and substitute. Write ICE table (or equivalent). 4. Solve the problem.

Percent ionization EQUILIBRIUM! INITIAL!

Barbituric acid (Ka = 1. 1 x 10 -4) is used in the manufacture of some sedatives. For a 0. 673 M solution of barbituric acid calculate the % ionization. Answer: 1. 28%

Penicillin, an antibotic often used to treat bacterial infection, is a weak acid. Its Ka is 1. 7 x 10 -3. Calculate the p. H of a 0. 187 M solution of penicillin. Answer: 1. 77

An unknown weak acid, HA, is found to ionize 4. 67% in a solution originally 0. 125 M. What is the value of Ka? Answer: 2. 86 x 10 -4

Weak Acids

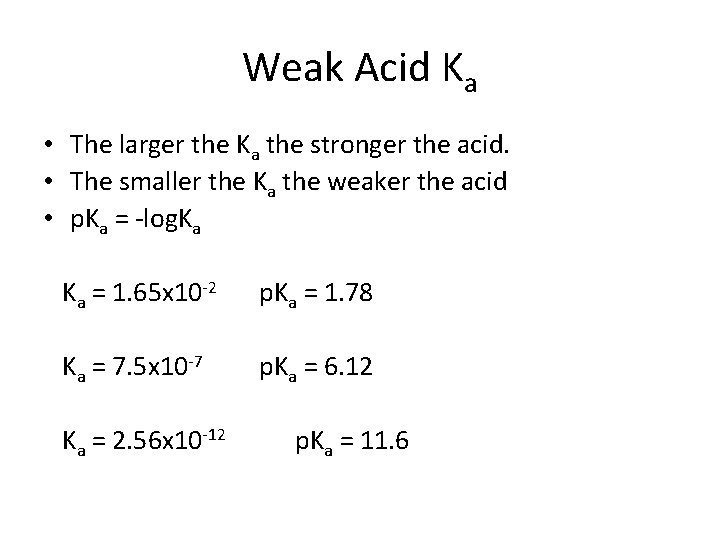
Weak Acid Ka • The larger the Ka the stronger the acid. • The smaller the Ka the weaker the acid • p. Ka = -log. Ka Ka = 1. 65 x 10 -2 p. Ka = 1. 78 Ka = 7. 5 x 10 -7 p. Ka = 6. 12 Ka = 2. 56 x 10 -12 p. Ka = 11. 6

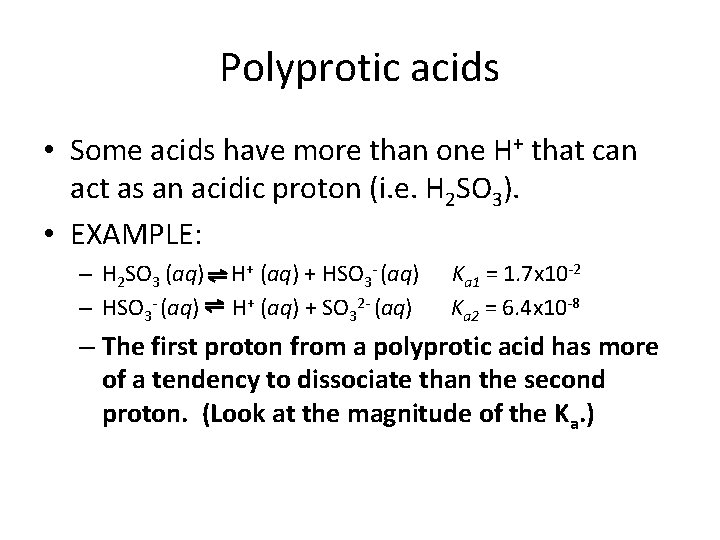
Polyprotic acids • Some acids have more than one H+ that can act as an acidic proton (i. e. H 2 SO 3). • EXAMPLE: – H 2 SO 3 (aq) – HSO 3 - (aq) H+ (aq) + SO 32 - (aq) Ka 1 = 1. 7 x 10 -2 Ka 2 = 6. 4 x 10 -8 – The first proton from a polyprotic acid has more of a tendency to dissociate than the second proton. (Look at the magnitude of the Ka. )

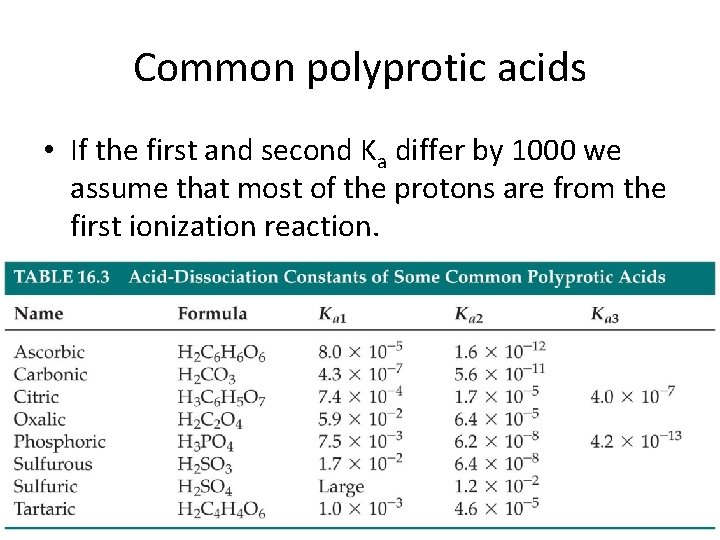
Common polyprotic acids • If the first and second Ka differ by 1000 we assume that most of the protons are from the first ionization reaction.
![Determine the H the p H and the PO 43 in an aqueous Determine the [H+], the p. H and the [PO 43 -] in an aqueous](https://slidetodoc.com/presentation_image/4f0791ed9f383eebd2df77ac06af0f48/image-22.jpg)
Determine the [H+], the p. H and the [PO 43 -] in an aqueous solution of 0. 100 M H 3 PO 4. Ka 1 = 7. 5 x 10 -3 Ka 2 = 6. 2 x 10 -8 Ka 3 = 4. 2 x 10 -13
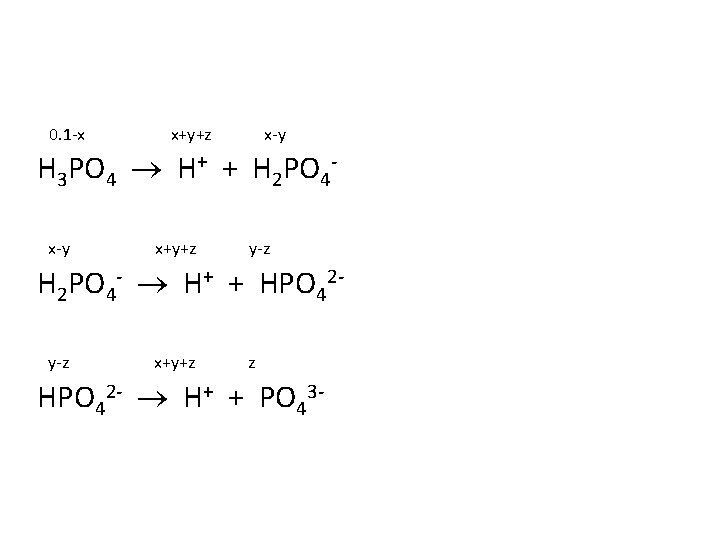
0. 1 -x x+y+z x-y H 3 PO 4 H+ + H 2 PO 4 x-y x+y+z y-z H 2 PO 4 - H+ + HPO 42 y-z x+y+z z HPO 42 - H+ + PO 43 -
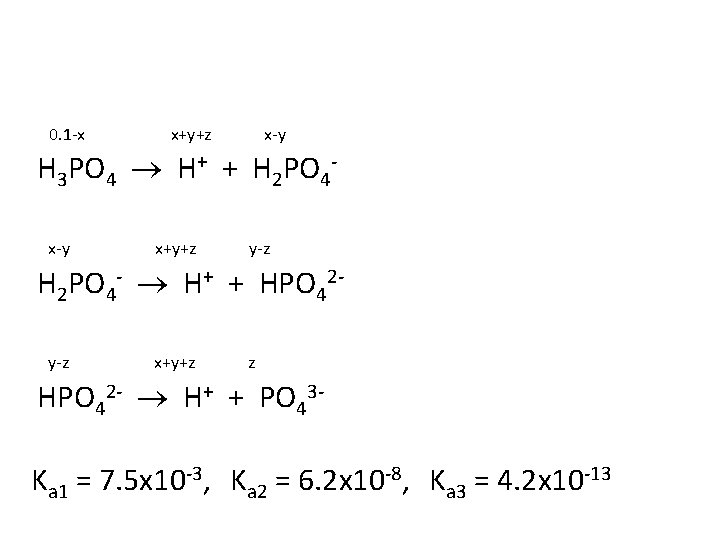
0. 1 -x x+y+z x-y H 3 PO 4 H+ + H 2 PO 4 x-y x+y+z y-z H 2 PO 4 - H+ + HPO 42 y-z x+y+z z HPO 42 - H+ + PO 43 Ka 1 = 7. 5 x 10 -3, Ka 2 = 6. 2 x 10 -8, Ka 3 = 4. 2 x 10 -13
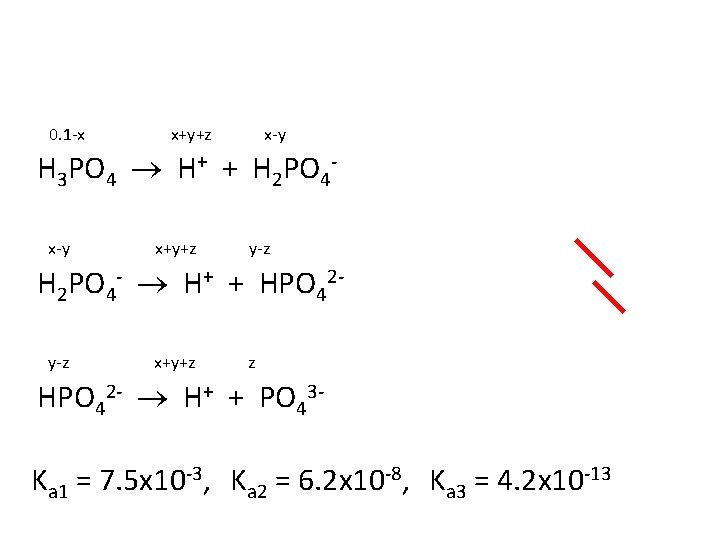
0. 1 -x x+y+z x-y H 3 PO 4 H+ + H 2 PO 4 x-y x+y+z y-z H 2 PO 4 - H+ + HPO 42 y-z x+y+z z HPO 42 - H+ + PO 43 Ka 1 = 7. 5 x 10 -3, Ka 2 = 6. 2 x 10 -8, Ka 3 = 4. 2 x 10 -13
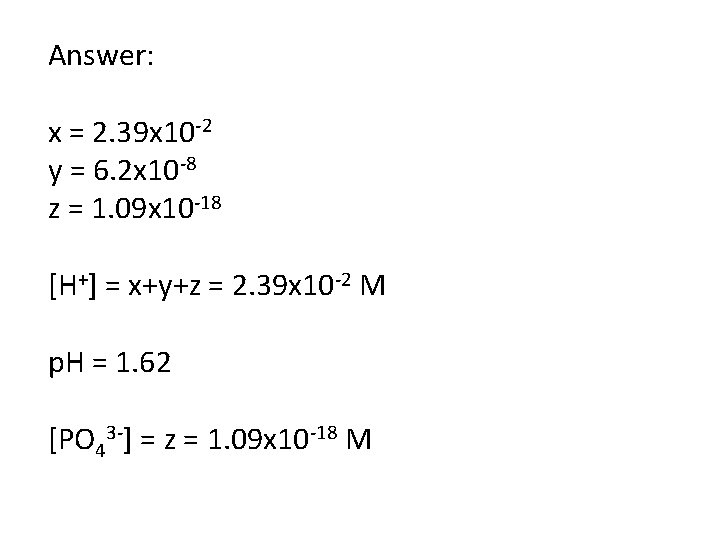
Answer: x = 2. 39 x 10 -2 y = 6. 2 x 10 -8 z = 1. 09 x 10 -18 [H+] = x+y+z = 2. 39 x 10 -2 M p. H = 1. 62 [PO 43 -] = z = 1. 09 x 10 -18 M

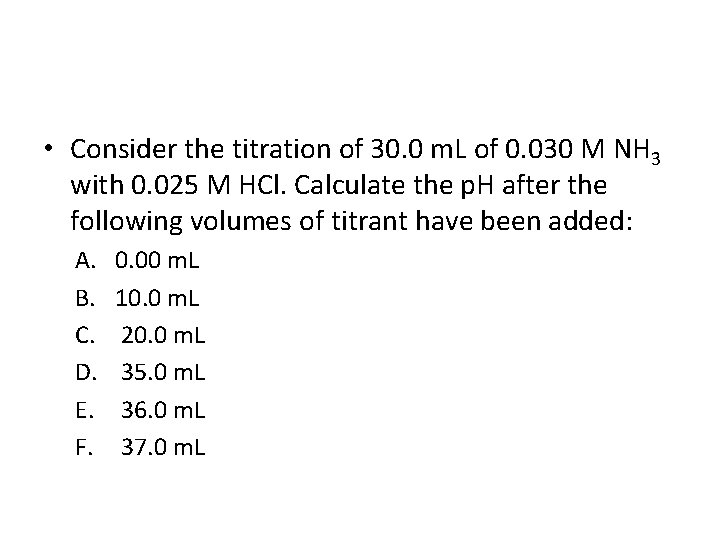
• Consider the titration of 30. 0 m. L of 0. 030 M NH 3 with 0. 025 M HCl. Calculate the p. H after the following volumes of titrant have been added: A. B. C. D. E. F. 0. 00 m. L 10. 0 m. L 20. 0 m. L 35. 0 m. L 36. 0 m. L 37. 0 m. L
• Calculate the p. H of the solution formed when 45. 0 m. L of 0. 100 M Na. OH is added to 50. 0 m. L of 0. 100 M HC 2 H 3 O 3 (Ka = 1. 8*10 -5).