We usually think of reducing equivalents as products
















- Slides: 105


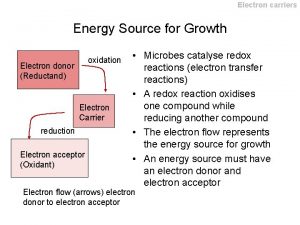
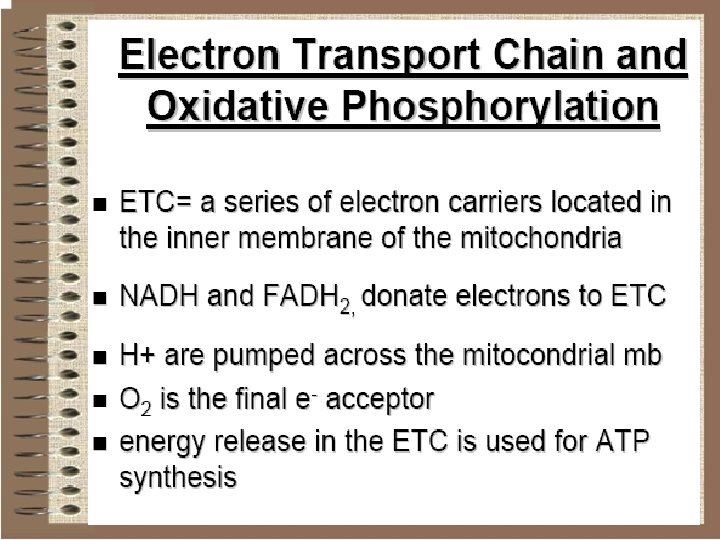
We usually think of reducing equivalents as products of glycolysis and the citric acid cycle since the oxidation of glucose and acetyl Co. A is coupled to the reduction of NADH and Q. In this chapter we learn that the subsequent reoxidation of NADH and results in the passage of electrons through a membrane-associated electron transport system where the energy released can be saved through the phosphorylation of ADP to ATP. The electrons are eventually passed to a terminal electron acceptor. In most species this terminal electron acceptor is molecular oxygen which is why the overall process is often called oxidative phosphorylation. The combined pathway of electron transport and ATP synthesis involves numerous enzymes and coenzymes. It also depends absolutely on the presence of a membrane compartment since one of the key steps in coupling electron transport to ATP synthesis involves the creation of a p. H gradient across a membrane. In eukaryotes, the membrane is the inner mitochondrial membrane and in prokaryotes it is the plasma membrane.

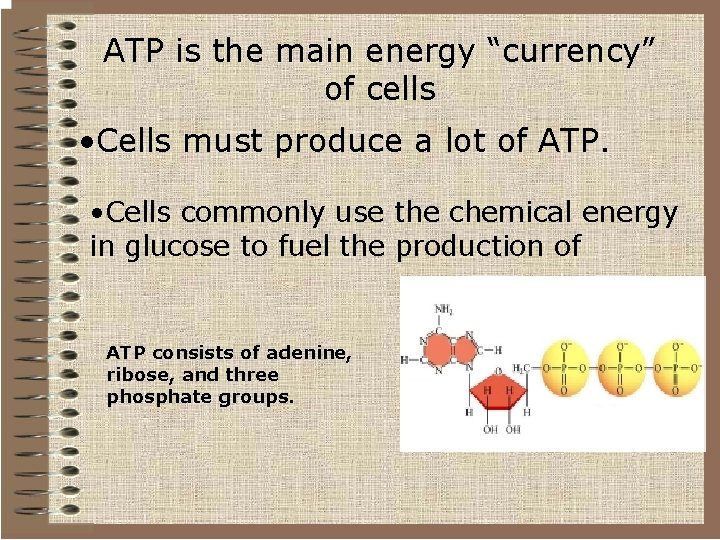
ATP is the main energy “currency” of cells • Cells must produce a lot of ATP. • Cells commonly use the chemical energy in glucose to fuel the production of ATP consists of adenine, ribose, and three phosphate groups.

Overview of Membrane-associated Electron Transport and ATP Synthesis In the complete pathway, electrons are passed from NADH to the terminal electron acceptor. There are many different terminal electron acceptors but we are mostly interested in the pathway found in eukaryotic mitochondria where molecular oxygen is reduced to form water. As electrons pass along the electron transport chain from NADH to the energy they release is used to transfer protons from inside the mitochondrion to the intermembrane space between the double membranes. This proton gradient is used to drive ATP synthesis in a reaction catalyzed by ATP synthase

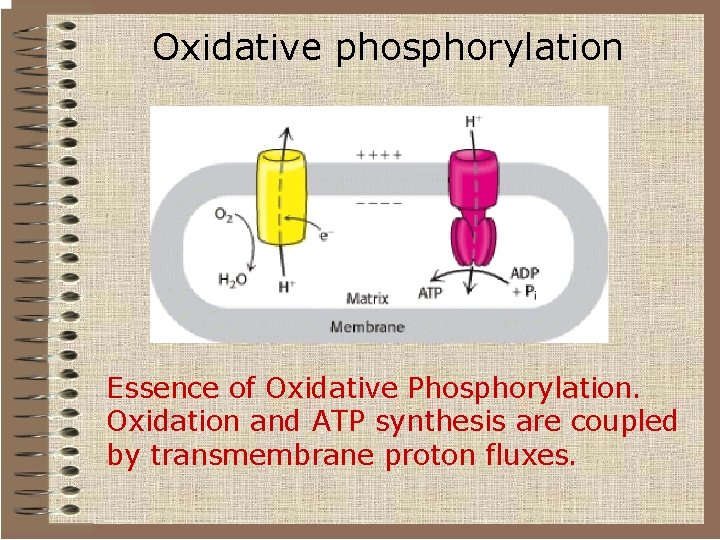
Oxidative phosphorylation • Ox. Phos is the culmination of a series of energy transformations that are called cellular respiration. 1. Carbonfuels are oxidized in TCA cycle to yield electrons with high transfer potential 2. This electron-motive force is converted into a proton-motive force 3. Proton-motive force is converted into phosphoryl transfer potential

Oxidative phosphorylation Essence of Oxidative Phosphorylation. Oxidation and ATP synthesis are coupled by transmembrane proton fluxes.


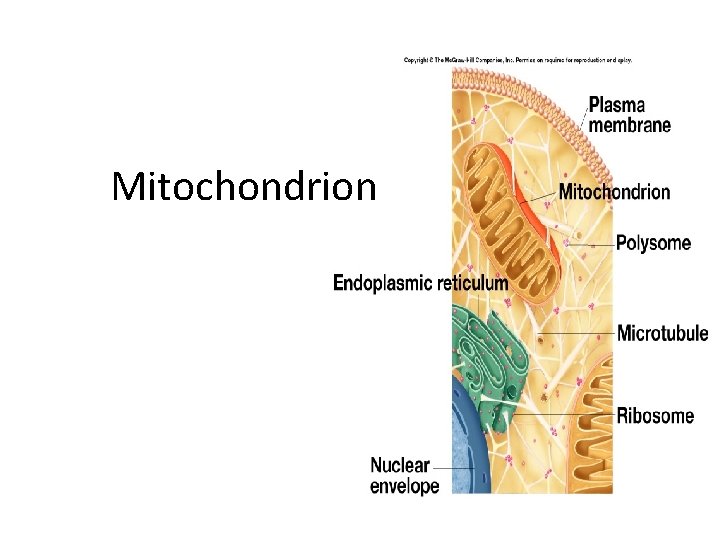
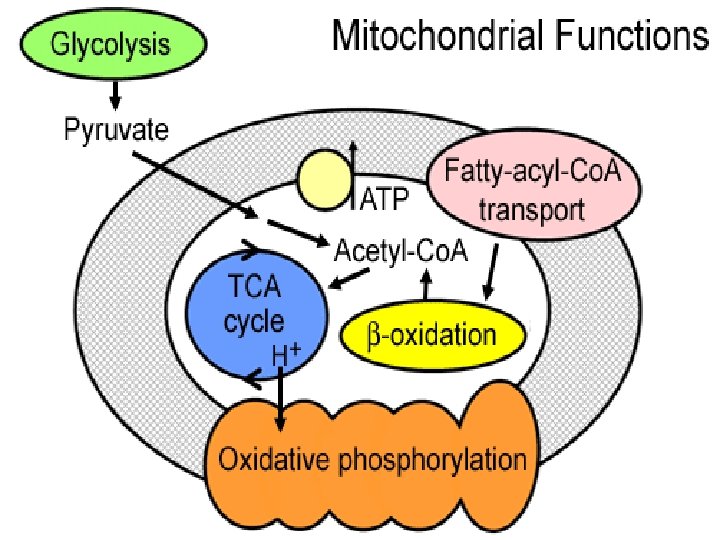
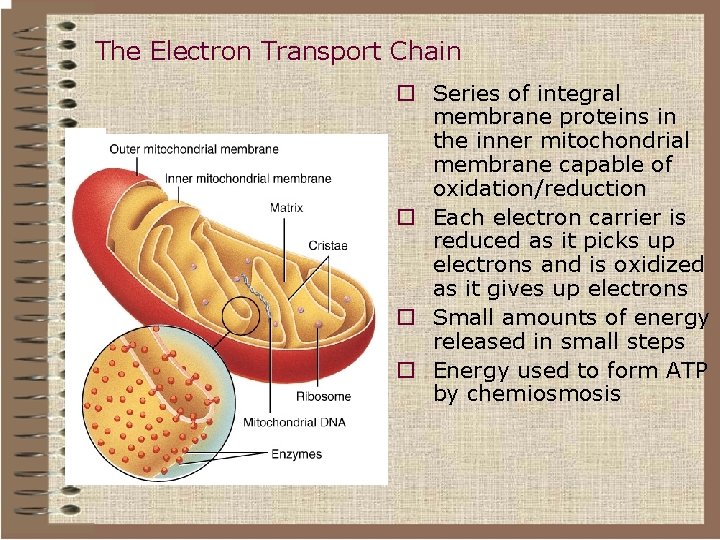
The Mitochondrion This organelle is the site of the citric acid cycle and fatty acid oxidation, both of which generate reduced coenzymes. The reduced coenzymes are oxidized by the electron transport complexes.

Mitochondrial Electron Transport • In 1948 Lehninger and Kennedy showed that oxidative phosphorylation occurred in the mitochondrion. • The electon transport chain is associated with the mitochondrial inner membrane

Mitochondrion

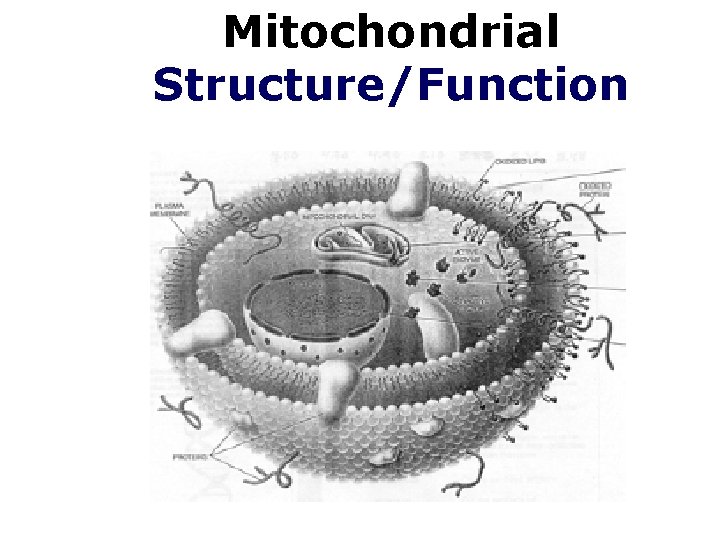
Mitochondrial Structure/Function

Molecular make up • Mostly protein, lipids to a lesser degree • Mitochondrial proteins are constantly renewed • Small quantities of DNA and RNA • Average half life of mitochondrial proteins in rat liver cell is 10 days

• Mitochondria • 15 -20% of liver cell volume • More in muscle • Associated – fatty acid/oil droplets • Function is intimately tied to membrane structure

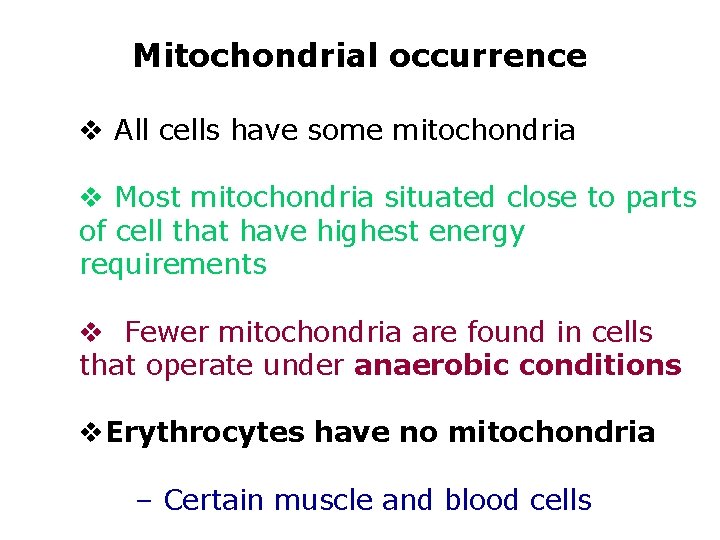
Mitochondrial occurrence v All cells have some mitochondria v Most mitochondria situated close to parts of cell that have highest energy requirements v Fewer mitochondria are found in cells that operate under anaerobic conditions v. Erythrocytes have no mitochondria – Certain muscle and blood cells

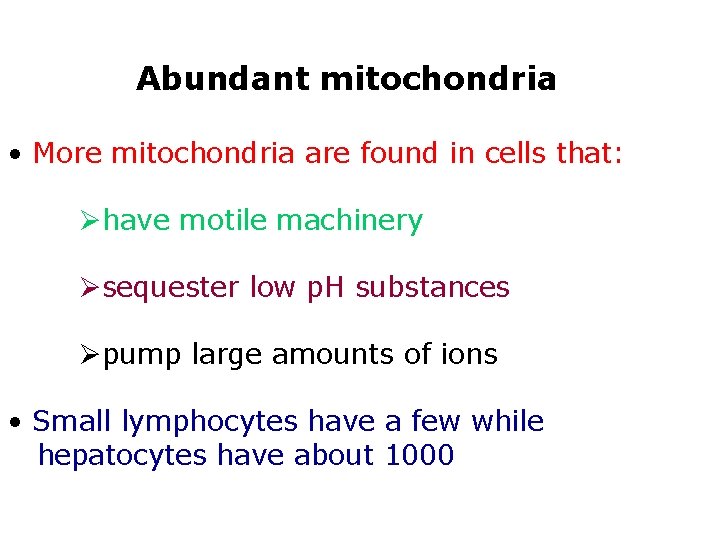
Abundant mitochondria • More mitochondria are found in cells that: Øhave motile machinery Øsequester low p. H substances Øpump large amounts of ions • Small lymphocytes have a few while hepatocytes have about 1000

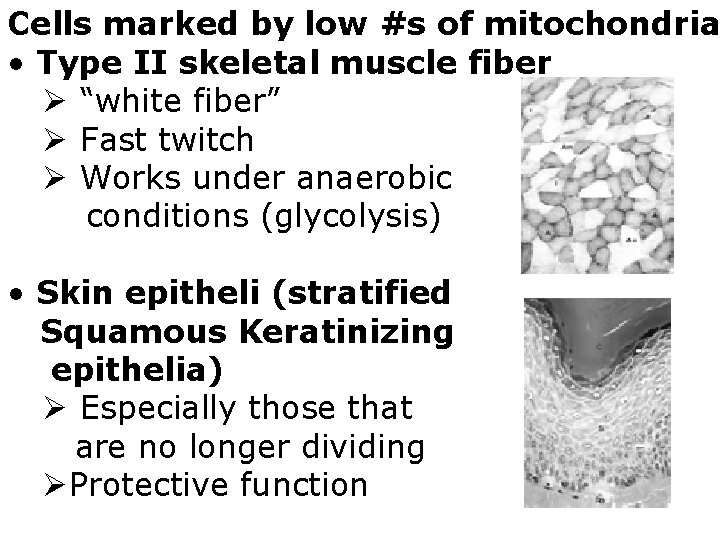
Cells marked by low #s of mitochondria • Type II skeletal muscle fiber Ø “white fiber” Ø Fast twitch Ø Works under anaerobic conditions (glycolysis) • Skin epitheli (stratified Squamous Keratinizing epithelia) Ø Especially those that are no longer dividing ØProtective function

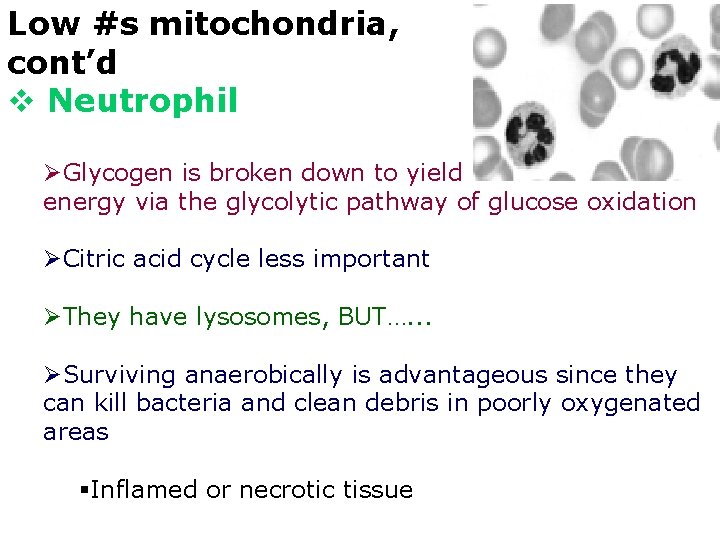
Low #s mitochondria, cont’d v Neutrophil ØGlycogen is broken down to yield energy via the glycolytic pathway of glucose oxidation ØCitric acid cycle less important ØThey have lysosomes, BUT…. . . ØSurviving anaerobically is advantageous since they can kill bacteria and clean debris in poorly oxygenated areas §Inflamed or necrotic tissue

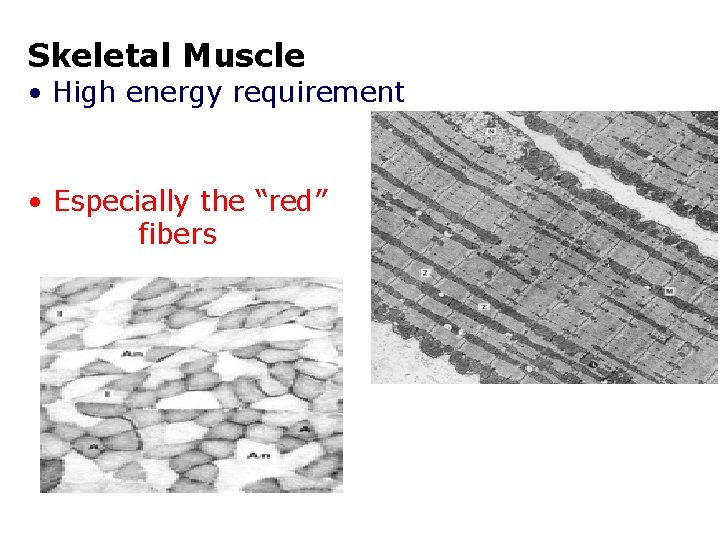
Skeletal Muscle • High energy requirement • Especially the “red” fibers

Ciliated cells • ATP is needed to move the microtubules in the cilia • Cilia helps move mucus along in the lumen of the trachea and the ovum in the oviduct

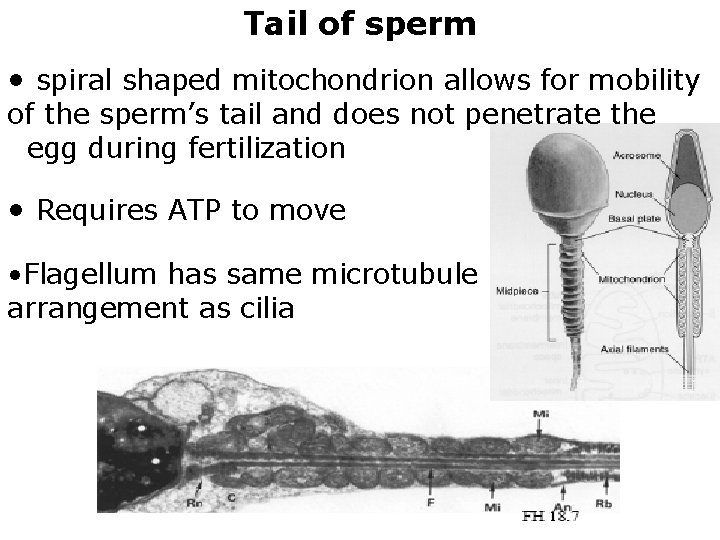
Tail of sperm • spiral shaped mitochondrion allows for mobility of the sperm’s tail and does not penetrate the egg during fertilization • Requires ATP to move • Flagellum has same microtubule arrangement as cilia

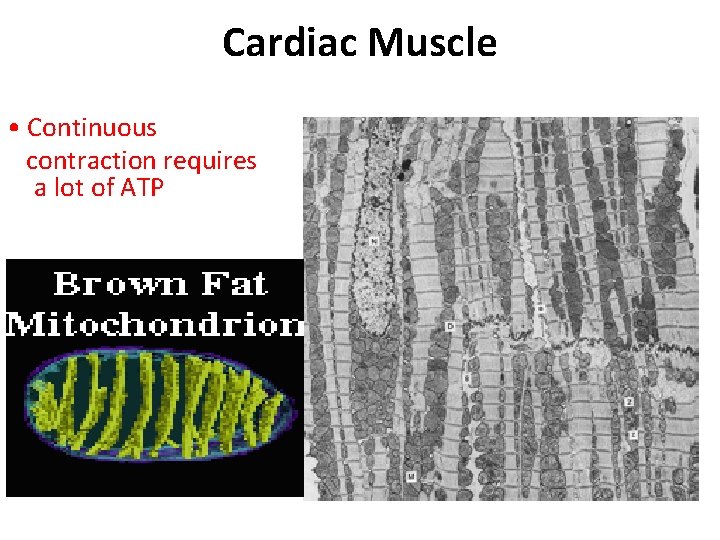
Cardiac Muscle • Continuous contraction requires a lot of ATP

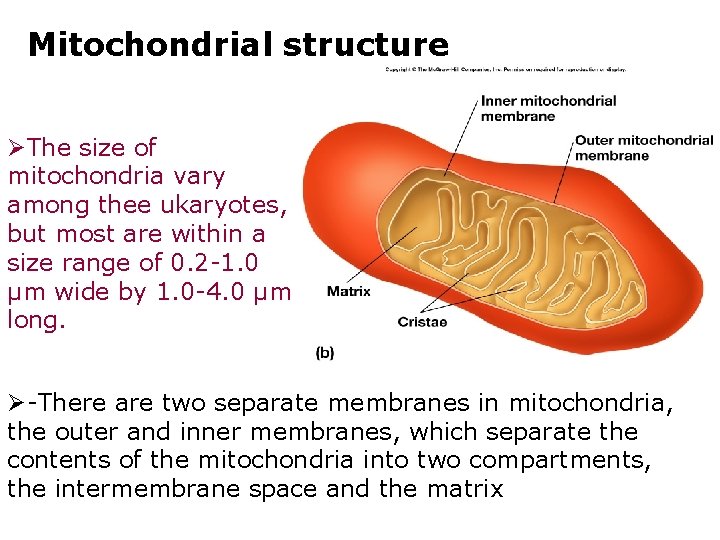
Mitochondrial structure ØThe size of mitochondria vary among thee ukaryotes, but most are within a size range of 0. 2 -1. 0 µm wide by 1. 0 -4. 0 µm long. Ø-There are two separate membranes in mitochondria, the outer and inner membranes, which separate the contents of the mitochondria into two compartments, the intermembrane space and the matrix

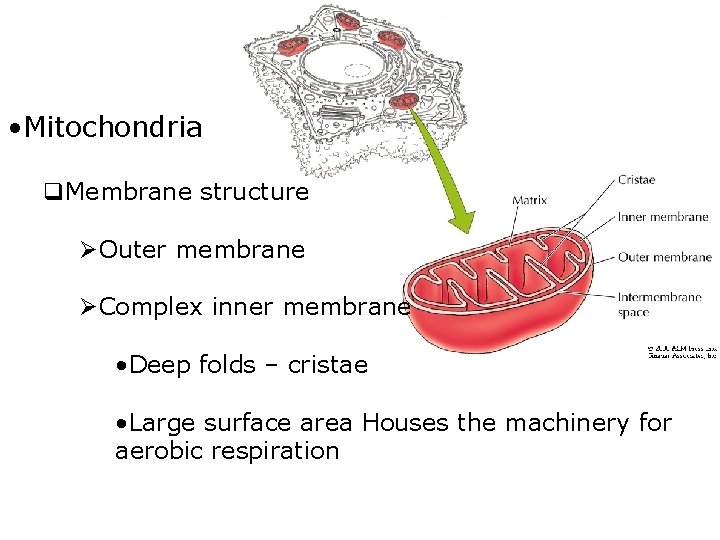
• Mitochondria q. Membrane structure ØOuter membrane ØComplex inner membrane • Deep folds – cristae • Large surface area Houses the machinery for aerobic respiration

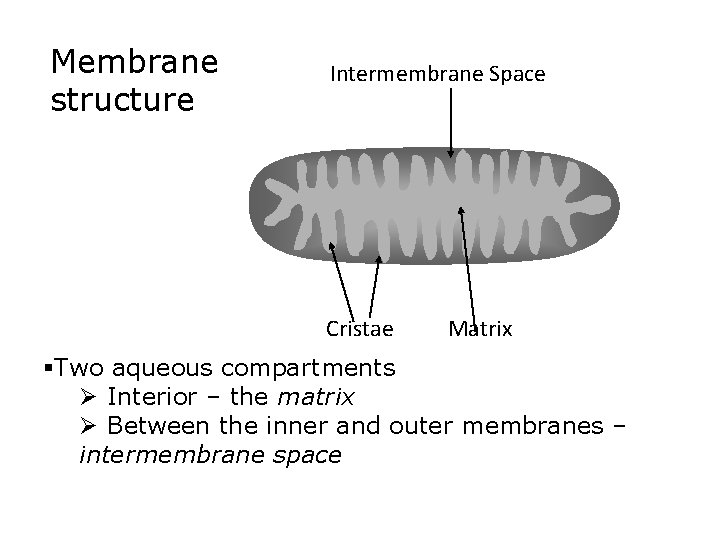
Membrane structure Intermembrane Space Cristae Matrix §Two aqueous compartments Ø Interior – the matrix Ø Between the inner and outer membranes – intermembrane space


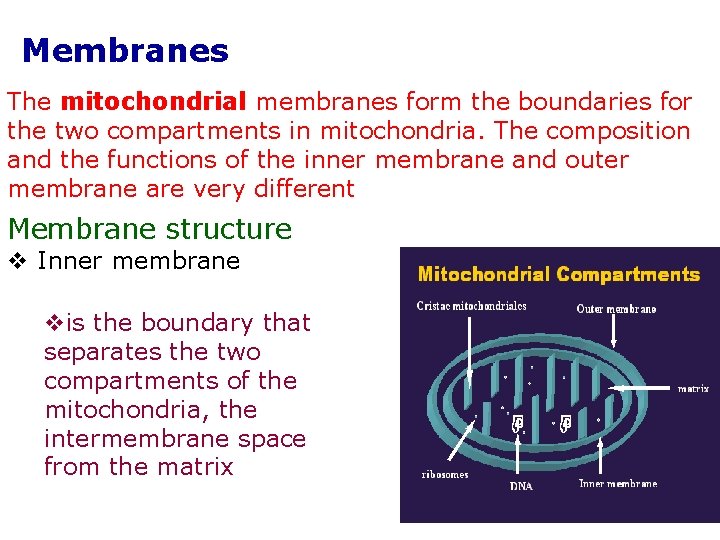
Membranes The mitochondrial membranes form the boundaries for the two compartments in mitochondria. The composition and the functions of the inner membrane and outer membrane are very different Membrane structure v Inner membrane vis the boundary that separates the two compartments of the mitochondria, the intermembrane space from the matrix

Ø lies adjacent to the outer membrane with extensive folding which form cristae that extend into the matrix Ø (80% protein) impermeable to most ions unless carried or transported. Ø> 100 different polypeptides ØHigh protein : lipid and low phospholipid : protein ØNo cholesterol Ømaintains electochemical gradient

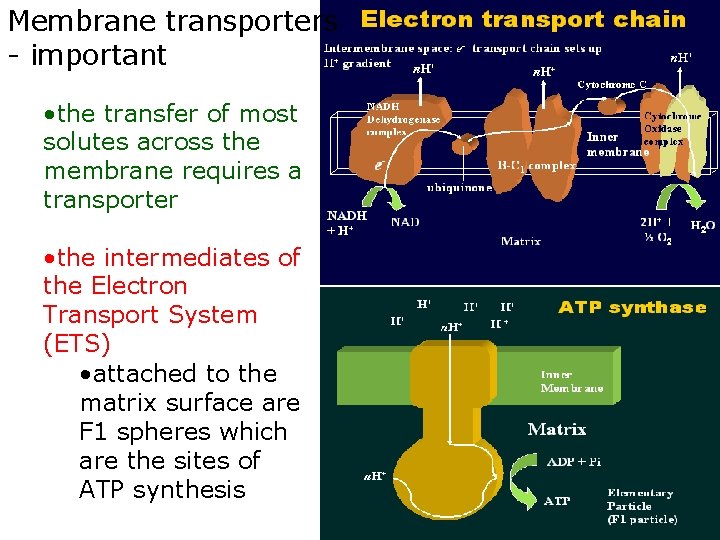
Membrane transporters - important • the transfer of most solutes across the membrane requires a transporter • the intermediates of the Electron Transport System (ETS) • attached to the matrix surface are F 1 spheres which are the sites of ATP synthesis

Membrane structure v. Outer membrane • the outer boundary of the mitochondria that separates the cytosol from the intermembrane space. • The membrane has a high phospholipid to protein ratio, approximately 0. 9. Among the proteins associated with the membrane are enzymes of fatty acid synthesis, nucleoside diphosphokinase and porin. • The membrane contain channels formed by porin which allow the free passage of molecules and ion with a molecular weight below about 5000 daltons (ATP, NAD (< 1, 000 Da)

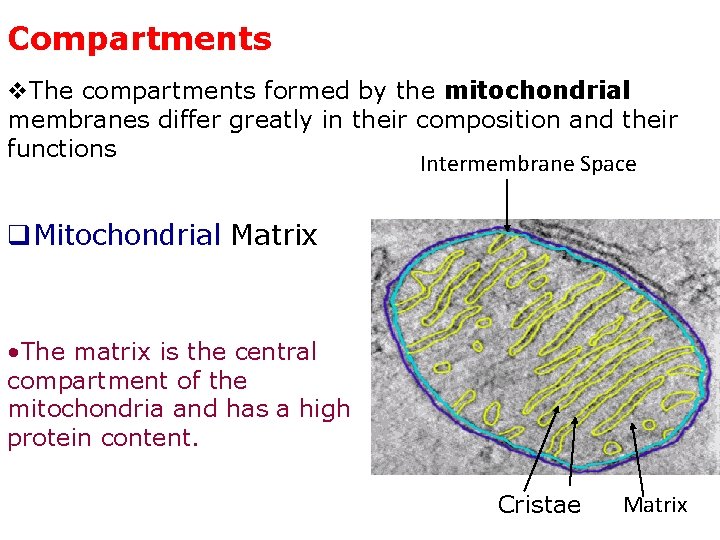
Compartments v. The compartments formed by the mitochondrial membranes differ greatly in their composition and their functions Intermembrane Space q. Mitochondrial Matrix • The matrix is the central compartment of the mitochondria and has a high protein content. Cristae Matrix

v. Among the proteins are the matrix • Pyruvate dehydrogenase • The enzymes of the tricarboxylic acid cycle • Enzymes of fatty acid oxidation • Enzymes required for RNA, DNA and protein synthesis v. The mitochondrial DNA (mit. DNA) & DNA – circular • Encodes mitochondrial polypeptides • Integral in inner membrane -Encodes two r-RNAs (12 S and 16 S) -Encodes 22 t-RNAs v The mitochondrial Ribosomes – smaller than in cytosol- are also located in matrix

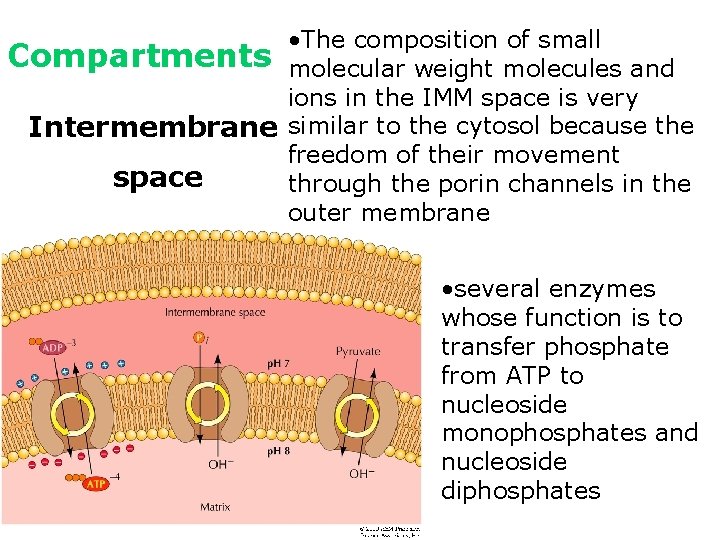
• The composition of small Compartments molecular weight molecules and ions in the IMM space is very Intermembrane similar to the cytosol because the freedom of their movement space through the porin channels in the outer membrane • several enzymes whose function is to transfer phosphate from ATP to nucleoside monophosphates and nucleoside diphosphates


Mitochondrial function Under aerobic conditions the primary function is to utilize free energy derived from oxidation of organic molecules to provide the energy to produce ATP. The reaction in the mitochondria may also serve as a route to interconvert molecules of the classes of biological molecules

Mitochondrial function • Oxidative phosphorylation Ø the process by which the enzymatic oxidation of cell metabolites is converted into ATP Ø Uses electrons from biochemical reactions Ø Occurs in a membrane bound electron transport system Ø Needs ATP-synthetase ü Uses H+ gradient to generate ATP

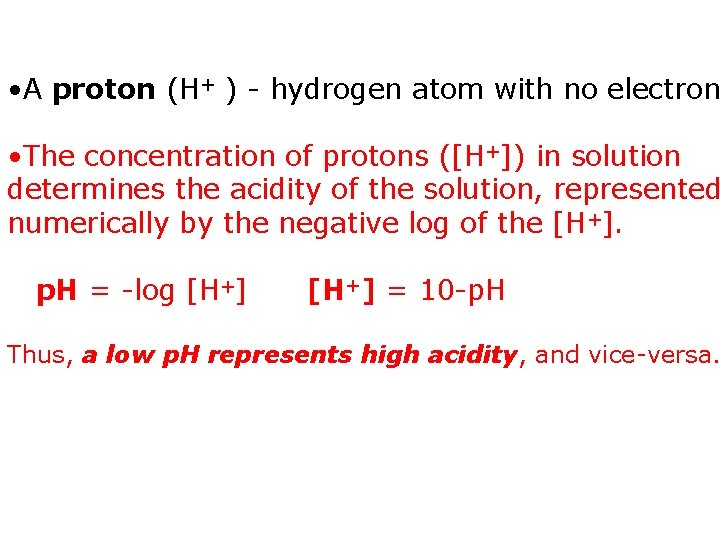
• A proton (H+ ) - hydrogen atom with no electron • The concentration of protons ([H+]) in solution determines the acidity of the solution, represented numerically by the negative log of the [H+]. p. H = -log [H+] = 10 -p. H Thus, a low p. H represents high acidity, and vice-versa.

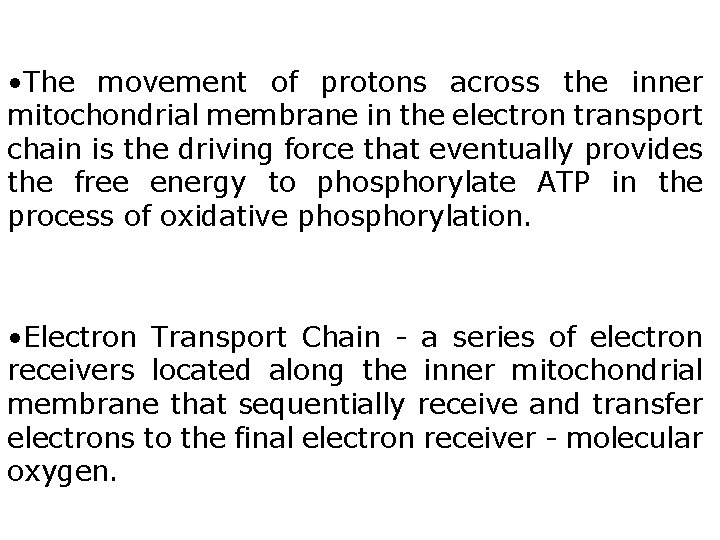
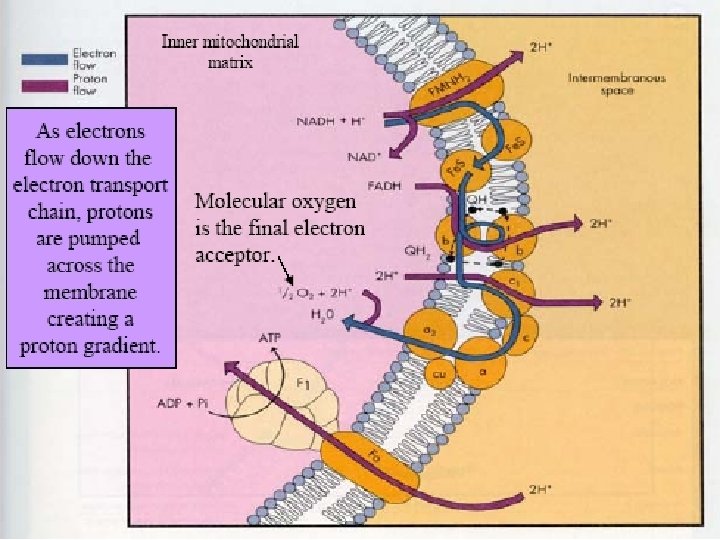
• The movement of protons across the inner mitochondrial membrane in the electron transport chain is the driving force that eventually provides the free energy to phosphorylate ATP in the process of oxidative phosphorylation. • Electron Transport Chain - a series of electron receivers located along the inner mitochondrial membrane that sequentially receive and transfer electrons to the final electron receiver - molecular oxygen.

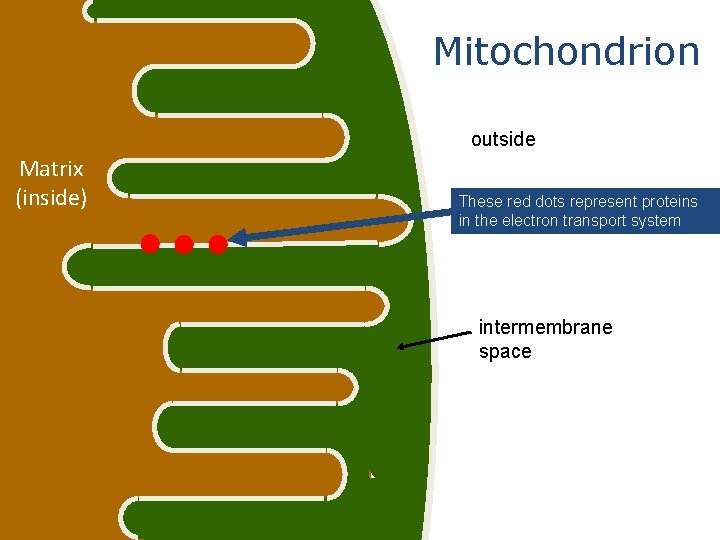
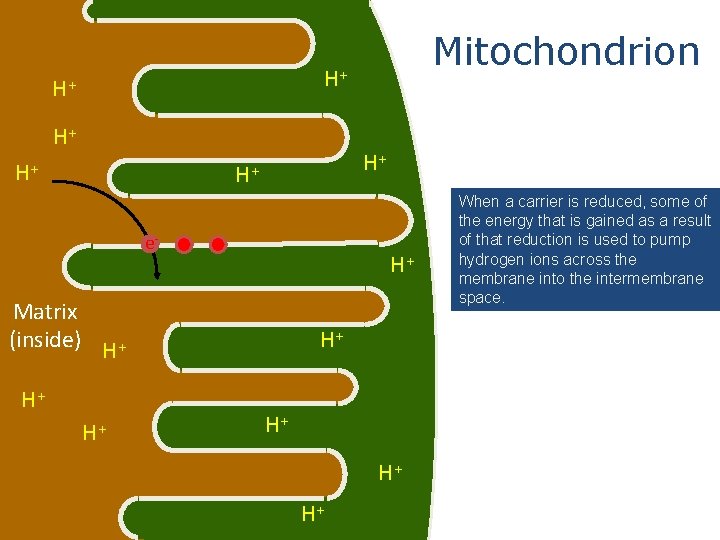
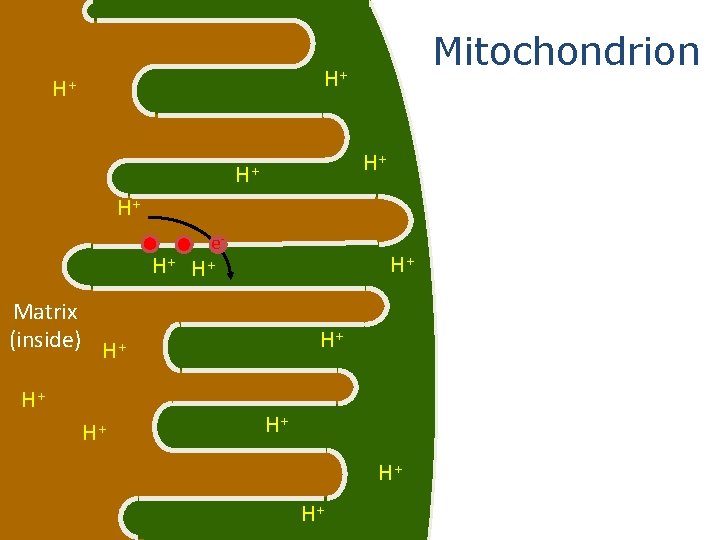
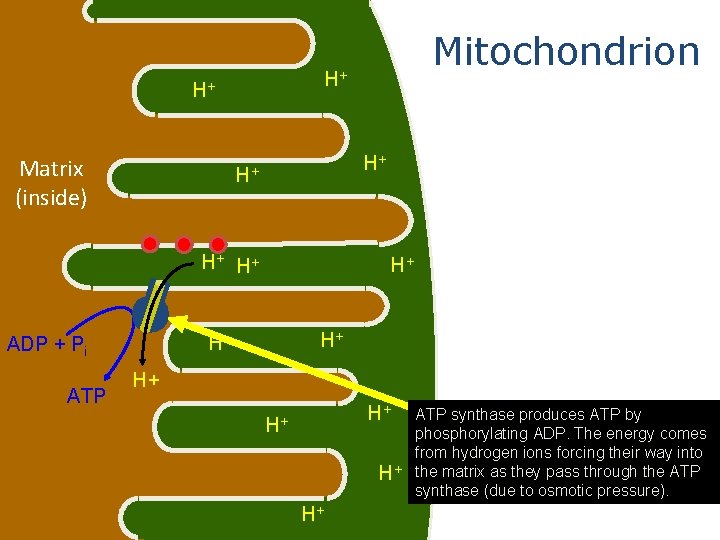
Mitochondrion outside Matrix (inside) These red dots represent proteins in the electron transport system intermembrane space

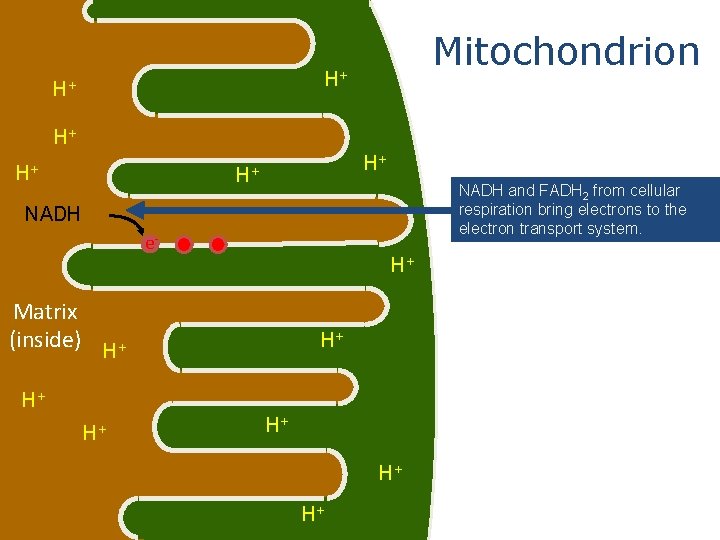
Mitochondrion H+ H+ H+ NADH and FADH 2 from cellular respiration bring electrons to the electron transport system. NADH e- H+ Matrix (inside) H+ H+

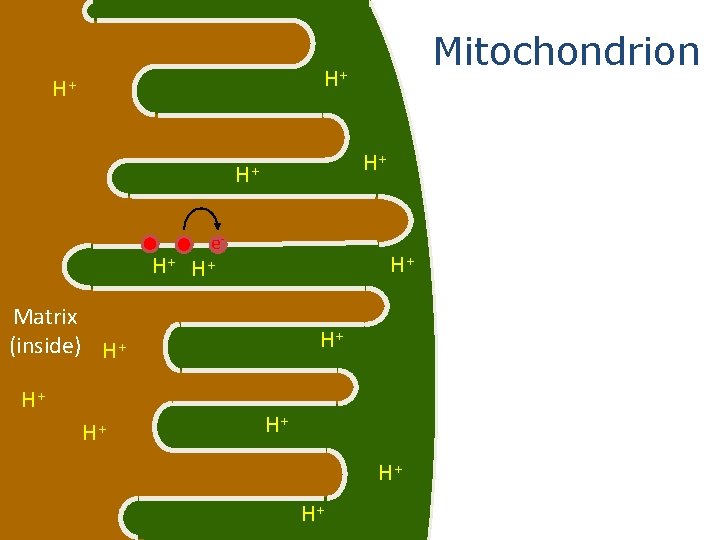
Mitochondrion H+ H+ H+ e- H+ Matrix (inside) H+ H+ When a carrier is reduced, some of the energy that is gained as a result of that reduction is used to pump hydrogen ions across the membrane into the intermembrane space.

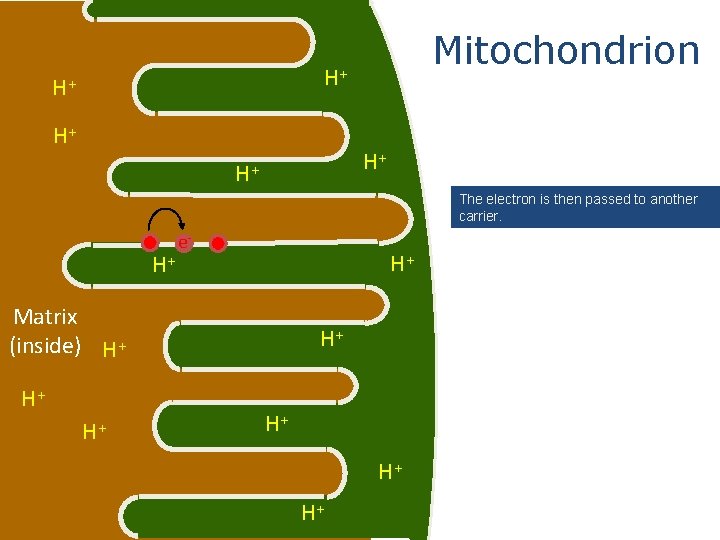
Mitochondrion H+ H+ H+ The electron is then passed to another carrier. e- H+ H+ Matrix (inside) H+ H+

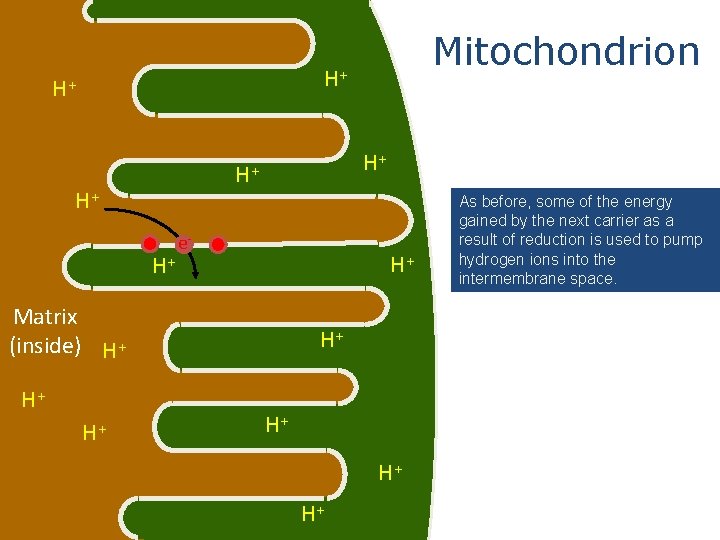
Mitochondrion H+ H+ H+ e- H+ H+ Matrix (inside) H+ H+ As before, some of the energy gained by the next carrier as a result of reduction is used to pump hydrogen ions into the intermembrane space.

Mitochondrion H+ H+ e- H+ H+ H+ Matrix (inside) H+ H+

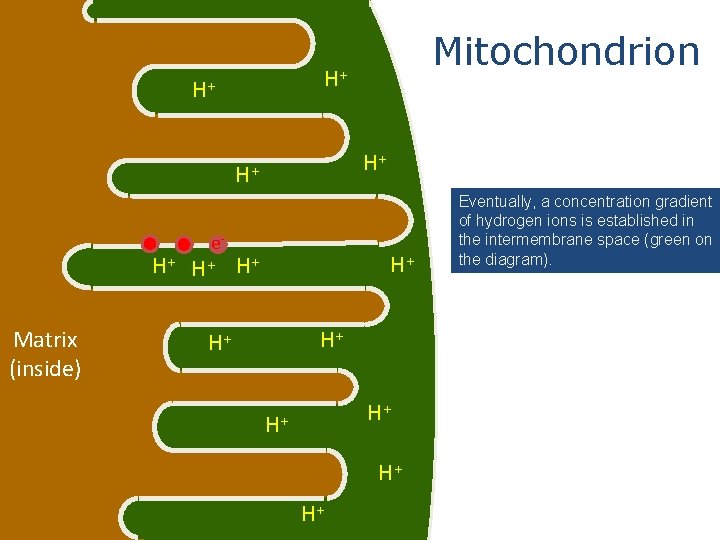
Mitochondrion H+ H+ H+ e- H+ H+ H+ Matrix (inside) H+ H+

H+ H+ e. H+ H+ Matrix (inside) Mitochondrion H+ H+ Eventually, a concentration gradient of hydrogen ions is established in the intermembrane space (green on the diagram).

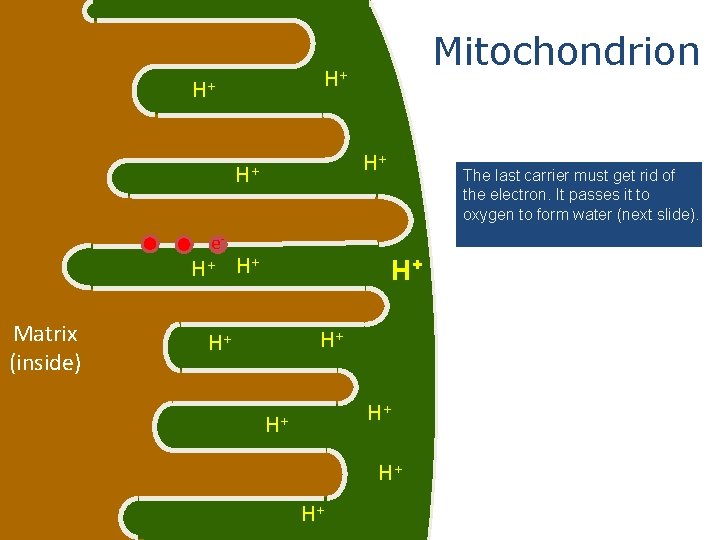
H+ H+ e. H+ Matrix (inside) Mitochondrion The last carrier must get rid of the electron. It passes it to oxygen to form water (next slide). H+ H+

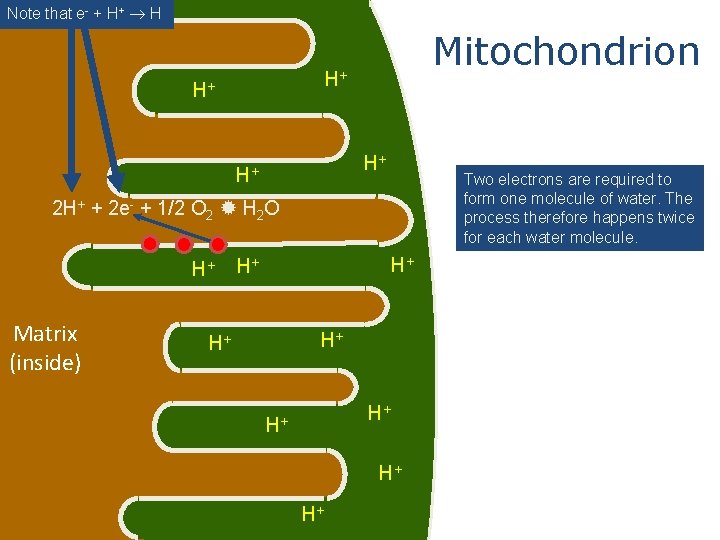
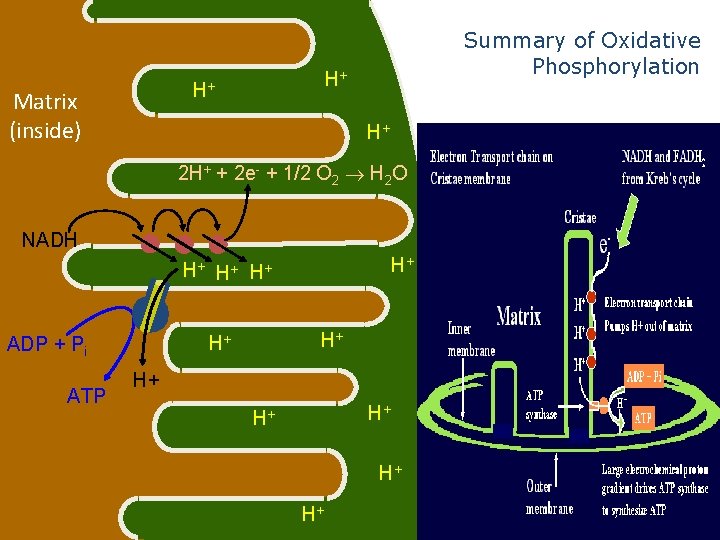
Note that e- + H+ H Mitochondrion H+ H+ Two electrons are required to form one molecule of water. The process therefore happens twice for each water molecule. 2 H+ + 2 e- + 1/2 O 2 H 2 O H+ Matrix (inside) H+ H+

H+ H+ Matrix (inside) Mitochondrion H+ H+ H+ ADP + Pi ATP H+ H+ H+ ATP synthase produces ATP by phosphorylating ADP. The energy comes from hydrogen ions forcing their way into H+ the matrix as they pass through the ATP synthase (due to osmotic pressure). H+ H+

H+ H+ Matrix (inside) Summary of Oxidative Phosphorylation H+ 2 H+ + 2 e- + 1/2 O 2 H 2 O NADH H+ H+ ATP H+ H+ ADP + Pi H+ H+ H+


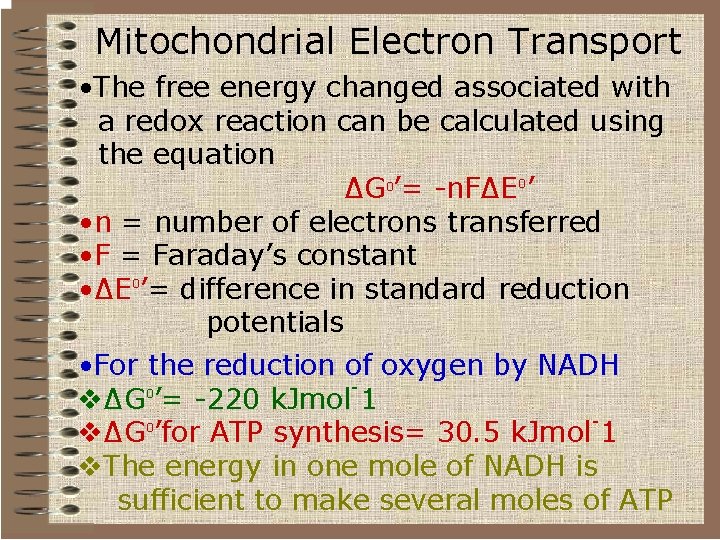
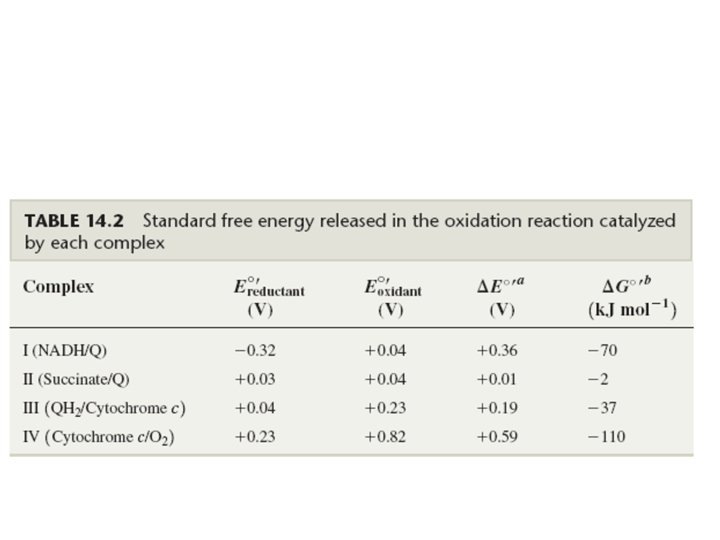
Mitochondrial Electron Transport • The free energy changed associated with a redox reaction can be calculated using the equation ∆G 0’= -n. F∆E 0’ • n = number of electrons transferred • F = Faraday’s constant • ∆E 0’= difference in standard reduction potentials • For the reduction of oxygen by NADH 0 v∆G ’= -220 k. Jmol 1 0 v∆G ’for ATP synthesis= 30. 5 k. Jmol 1 v. The energy in one mole of NADH is sufficient to make several moles of ATP

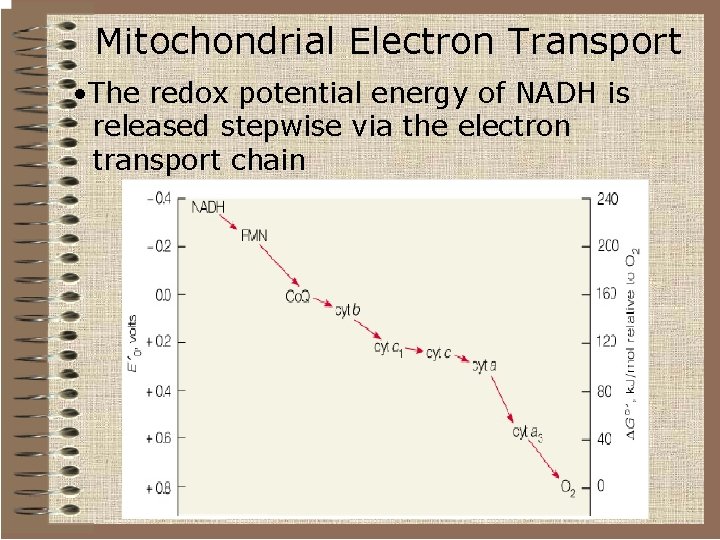
Mitochondrial Electron Transport • The redox potential energy of NADH is released stepwise via the electron transport chain

8 th home work Why do mitochondria have their own genome ?

Oxidative phosphorylation and The Chemiosmosis Hypothesis Several hypothesis have been put forward to explain how electron transport in mitochondria or bacterial membranes can be coupled to ATP formation.

The chemical coupling hypothesis suggests that oxidative phosphorylation is essentially identical to substrate level phosphorylation. That is , discreet energy rich intermediates are invovled.

The mechanochemical or conformational coupling model Oxidation – reduction reaction within the membrane result in an energized conformation of the membrane or in certain subunits of the membrane

The chemiosmosis hypothesis The electron carriers are asymetrically positioned within the membrane so that when H+ ions are produced they are released on only one side of the membrane. When H+ ions are utilized, they are obtained from the other side of the membrane. Thus the passage of 2 e_ down the electron transport system results in a ∆p. H across the inner membrane.

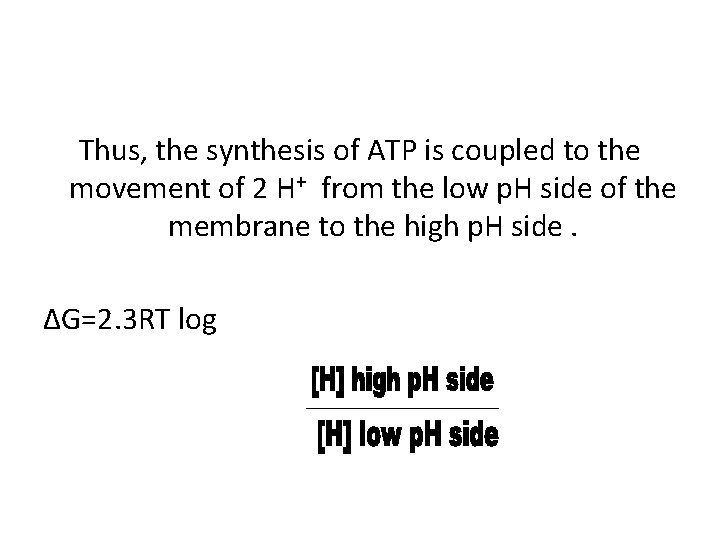
Thus, the synthesis of ATP is coupled to the movement of 2 H+ from the low p. H side of the membrane to the high p. H side. ∆G=2. 3 RT log


The Chemiosmotic Theory and the Protonmotive Force The chemiosmotic theory is the concept that a proton concentration gradient serves as the energy reservoir that drives ATP formation. The essential elements of this theory were originally formulated by Peter Mitchell in the early 1960 s. Many early attempts to identify an energy-rich phosphorylated metabolite that could transfer a phosphoryl group to ADP had ended in failure. Today, thanks to decades of work by many scientists, the formation and dissipation of ion gradients are acknowledged as a central motif in bioenergetics. Mitchell was awarded the Nobel Prize in Chemistry in 1978 for his contribution to our understanding of bioenergetics.


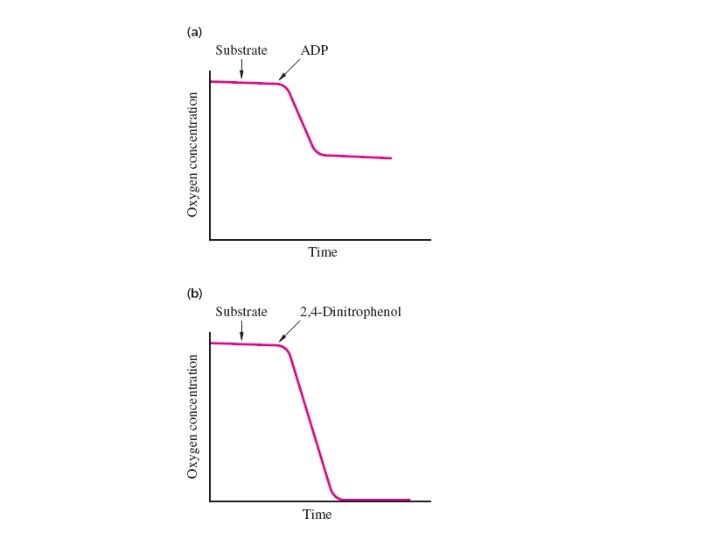
Historical Background: The Chemiosmotic Theory In 1956 Britton Chance and Ronald Williams hadshown that when intact isolated mitochondria are suspended in phosphate buffer they oxidize substrates and consume oxygen only when ADP is added to the suspension. In other words, the oxidation of a substrate must be coupled to the hosphorylation of ADP. Subsequent experiments showed that respiration proceeds rapidly until all the ADP has been phosphorylated and that the amount of consumed depends on the amount of ADP added.

Synthetic compounds called uncouplers stimulate the oxidation of substrates in the absence of ADP. The phenomenon of uncoupling helped show oxidation reactions are linked to ATP formation. In the presence of an uncoupler, oxygen uptake (respiration) proceeds until all the available oxygen is consumed. This rapid oxidation of substrates proceeds with little or no phosphorylation of ADP. In other words, these synthetic compounds uncouple oxidation from phosphorylation.


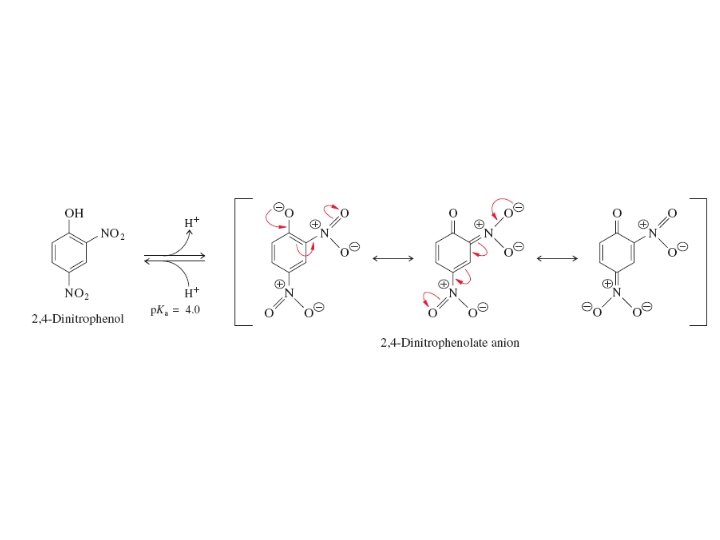
There are many different kinds of uncouplers and they are lipid-soluble weak acids. Both their protonated and conjugate base forms can cross the inner mitochondrial membrane; The anionic conjugate base retains lipid solubility because the negative charge is delocalized.


Mitchell proposed that the action of mitochondrial enzyme complexes generates a proton concentration gradient across the inner mitochondrial membrane. He suggested that this gradient provides the energy for ADP phosphorylation via an indirect coupling to electron transport. Mitchell’s ideas accounted for the effect of the lipid-soluble uncoupling agents —they bind protons in the cytosol, carry them through the inner membrane, and release them in the matrix, thereby dissipating the proton concentration gradient. The proton carriers uncouple electron transport (oxidation) from ATP synthesis because protons enter the matrix without passing through ATP synthase.

ATP synthase activity was first recognized in 1948 as ATPase activity in damaged mitochondria (i. e. , damaged mitochondria catalyze the hydrolysis of ATP to ADP and ). Most workers assumed that mitochondrial ATPase catalyzes the reverse reaction in undamaged mitochondria. This assumption proved to be correct. Efraim Racker and his coworkers isolated and characterized this membranebound oligomeric ATPase in the 1960 s. The proton-driven reversibility of the ATPase reaction was demonstrated by observing the expulsion of protons upon the hydrolysis of ATP in mitochondria

Further support came from experiments with small membrane vesicles where the enzyme was incorporated into the membrane. When a suitable proton gradient was created across the vesicle membrane, ATP was synthesized from ADP and Pi

9 th home work Explain why fever is accompanies the toxic overdoses of Aspirin drug ?

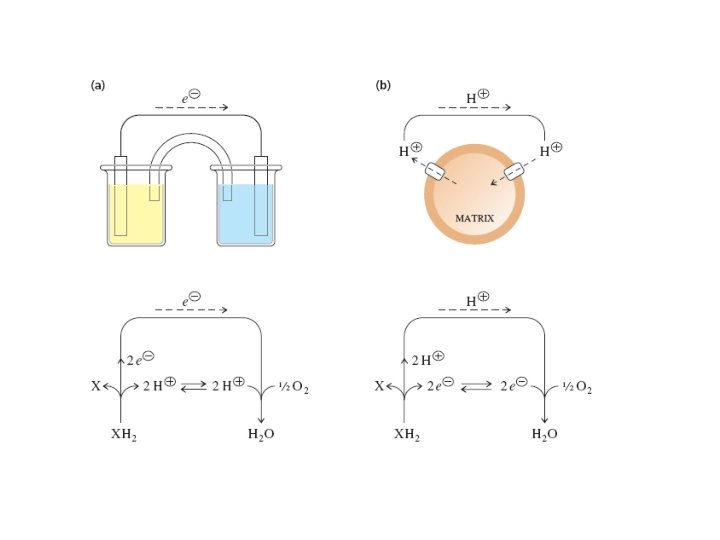
The Protonmotive Force • Protons are translocated into the intermembrane space by the membraneassociated electron transport complexes and they flow back into the matrix via ATP synthase. • This circular flow forms a circuit that is similar to an electrical circuit. The energy of the proton concentration gradient, called the protonmotive force, is analogous to the electromotive force of electrochemistry


1. In mitochondria, it is protons—not electrons—that flow through the external connection, an aqueous circuit connecting the membrane-associated electrontransport chain and ATP synthase. 2. The electrons still pass from the reducing agent to the oxidizing agent but in this case it is through the membraneassociated electron transport chain. 3. The free energy of these oxidation–reduction reactions is stored as the protonmotive force of the proton concentration gradient and is recovered in the phosphorylation of ADP.


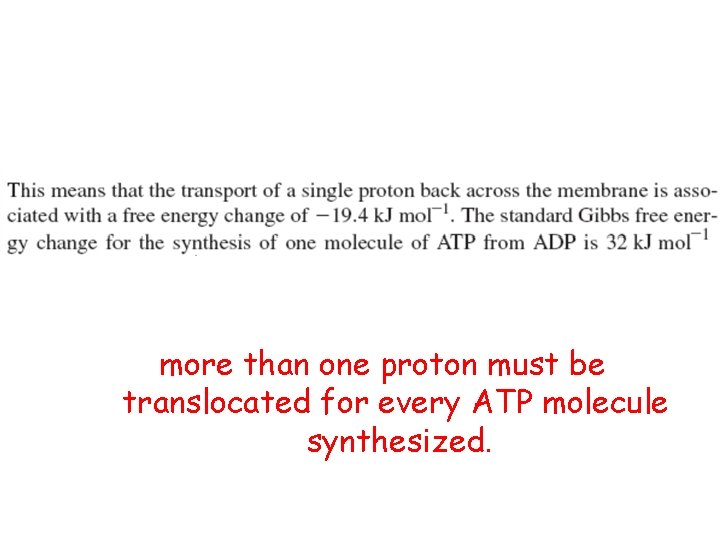
more than one proton must be translocated for every ATP molecule synthesized.

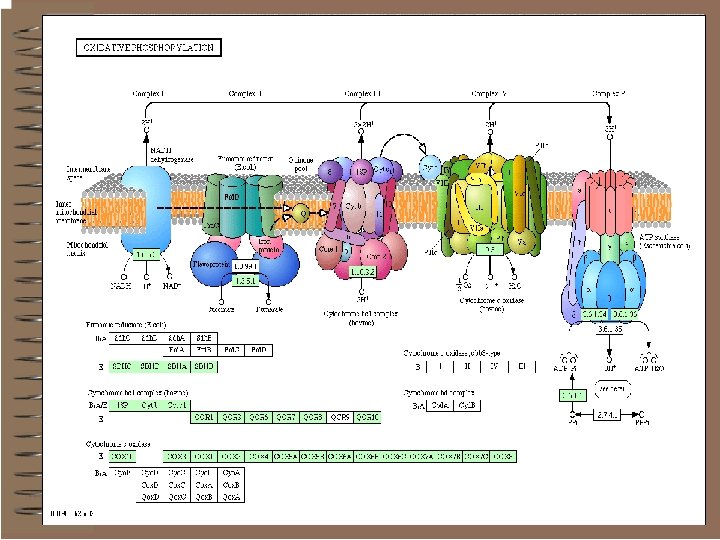
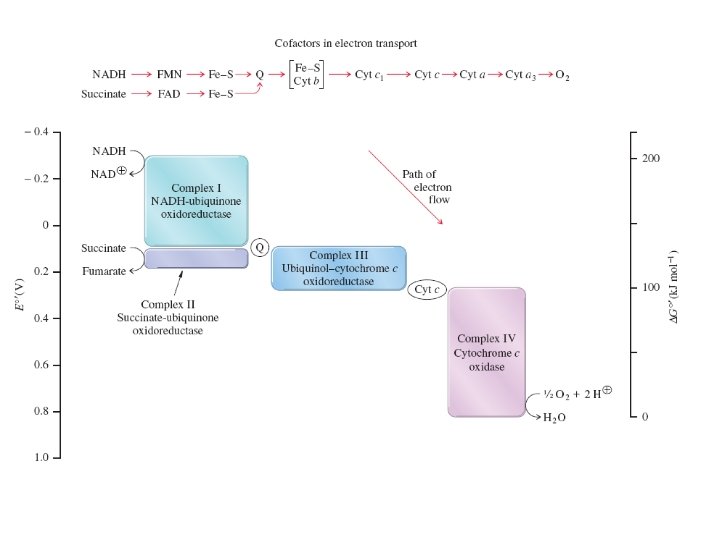
Complexes I Through IV The four enzyme complexes contain a wide variety of oxidation–reduction centers. These may be cofactors such as FAD, FMN, or ubiquinone (Q). Other centers include Fe–S clusters, protein coenzymes such as the heme-containing cytochromes, and copper proteins.

Electrons flow through the components of an electron transport chain in the direction of increasing reduction potential. The reduction potentials of each redox center fall between that of the strong reducing agent NADH and that of the terminal oxidizing agent The mobile coenzymes ubiquinone (Q) and cytochrome c serve as links between different complexes of the electron transport chain



The Electron Transport Chain o Series of integral membrane proteins in the inner mitochondrial membrane capable of oxidation/reduction o Each electron carrier is reduced as it picks up electrons and is oxidized as it gives up electrons o Small amounts of energy released in small steps o Energy used to form ATP by chemiosmosis

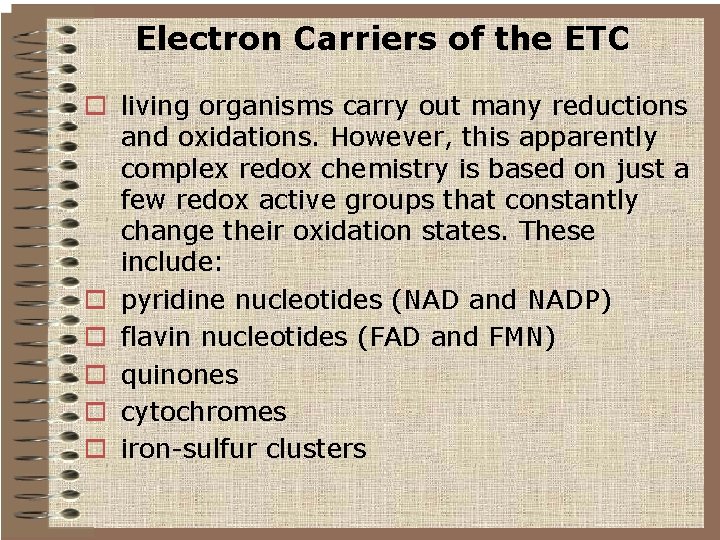
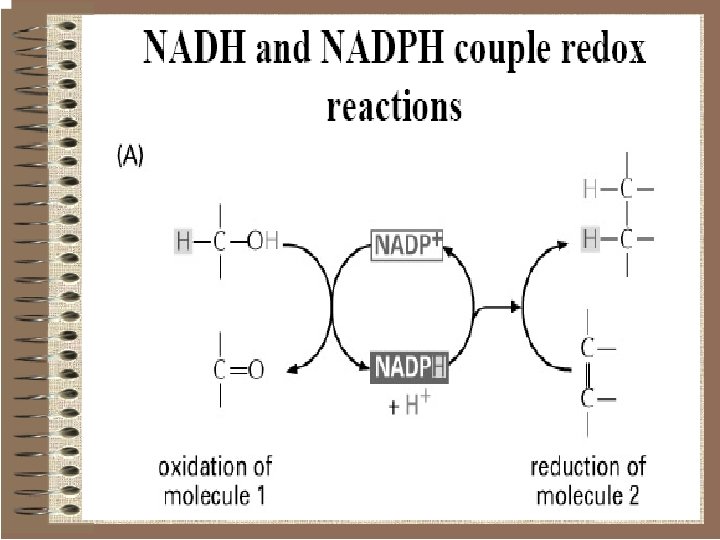
Electron Carriers of the ETC o living organisms carry out many reductions and oxidations. However, this apparently complex redox chemistry is based on just a few redox active groups that constantly change their oxidation states. These include: o pyridine nucleotides (NAD and NADP) o flavin nucleotides (FAD and FMN) o quinones o cytochromes o iron-sulfur clusters

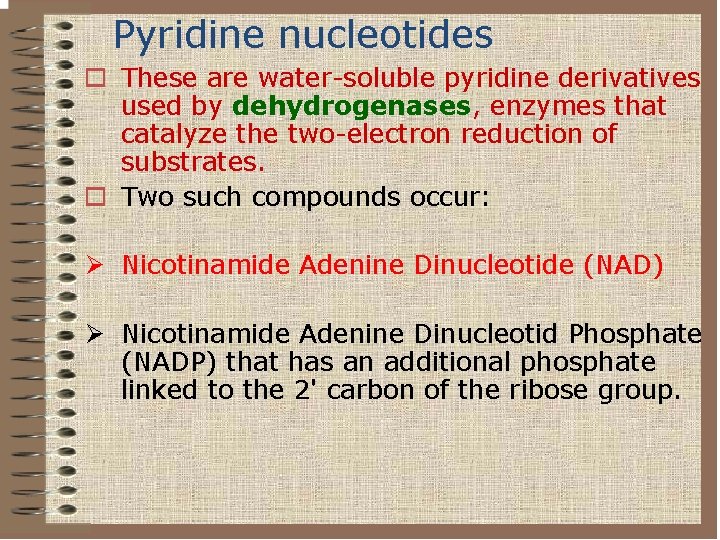
Pyridine nucleotides o These are water-soluble pyridine derivatives used by dehydrogenases, enzymes that catalyze the two-electron reduction of substrates. o Two such compounds occur: Ø Nicotinamide Adenine Dinucleotide (NAD) Ø Nicotinamide Adenine Dinucleotid Phosphate (NADP) that has an additional phosphate linked to the 2' carbon of the ribose group.

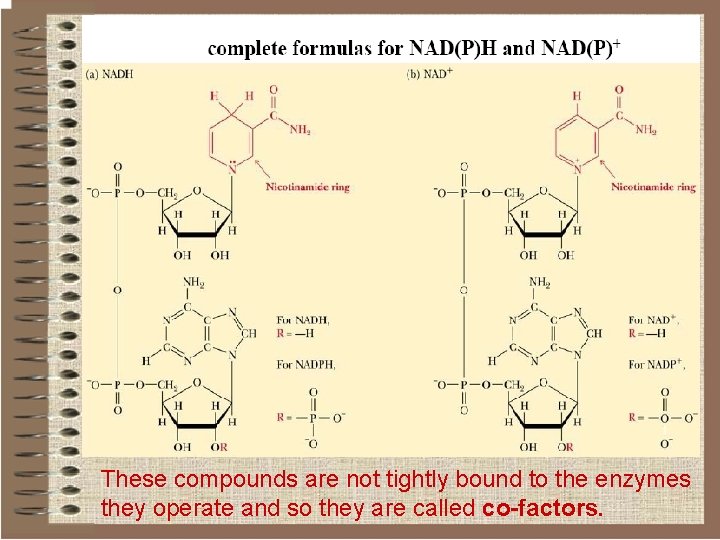
These compounds are not tightly bound to the enzymes they operate and so they are called co-factors.


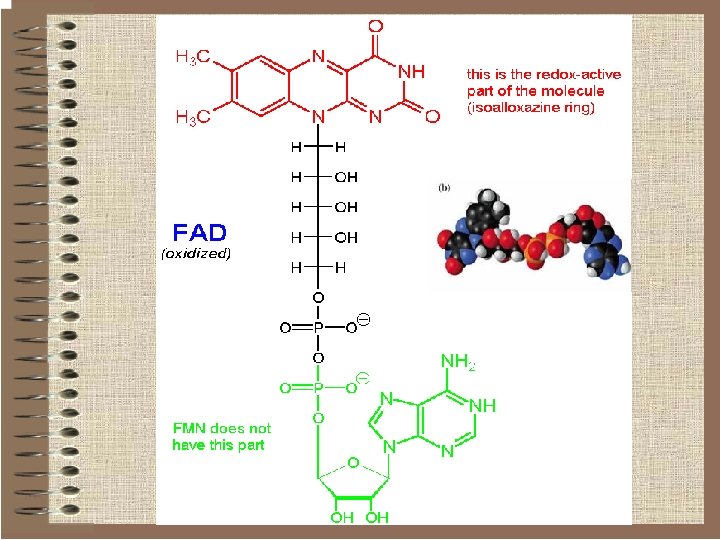
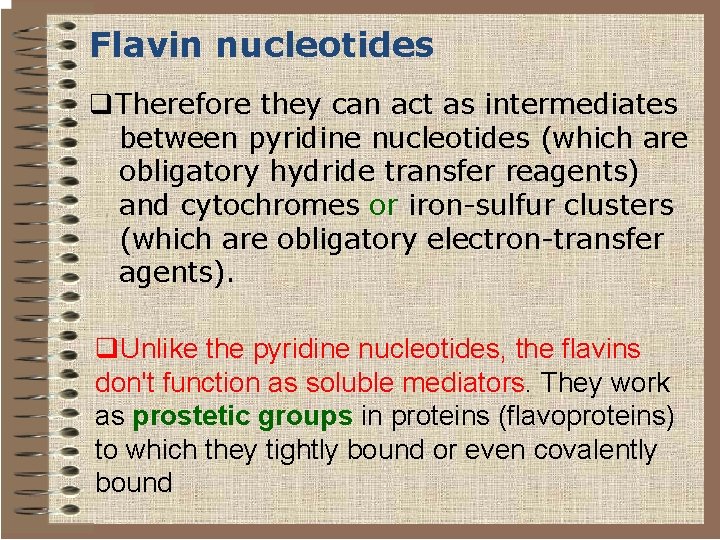
Flavin nucleotides o Some redox enzymes use redox groups derived from riboflavin; these are called flavoproteins. o The redox groups are the q Flavin Mononucleotide (FMN) q Flavin Adenine Dinucleotide (FAD).

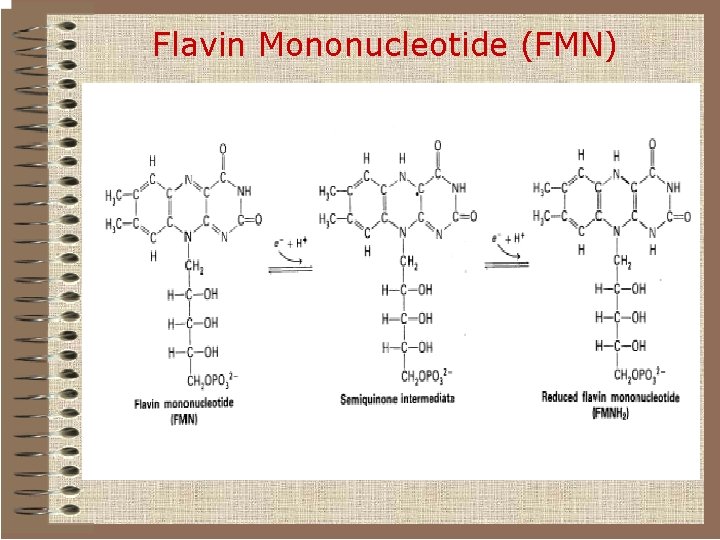
Flavin Mononucleotide (FMN)


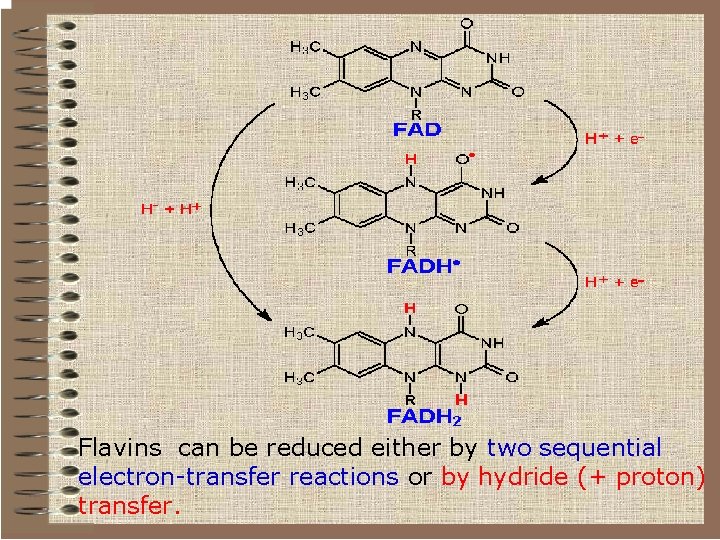
Flavins can be reduced either by two sequential electron-transfer reactions or by hydride (+ proton) transfer.

Flavin nucleotides q. Therefore they can act as intermediates between pyridine nucleotides (which are obligatory hydride transfer reagents) and cytochromes or iron-sulfur clusters (which are obligatory electron-transfer agents). q. Unlike the pyridine nucleotides, the flavins don't function as soluble mediators. They work as prostetic groups in proteins (flavoproteins) to which they tightly bound or even covalently bound

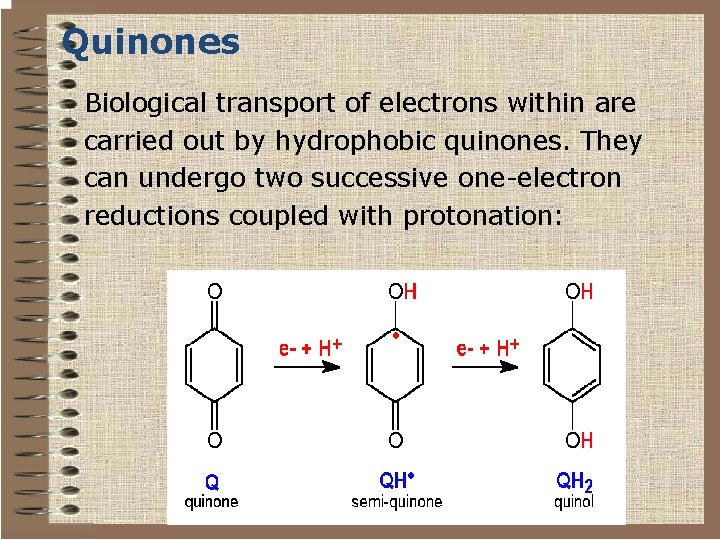
Quinones Biological transport of electrons within are carried out by hydrophobic quinones. They can undergo two successive one-electron reductions coupled with protonation:

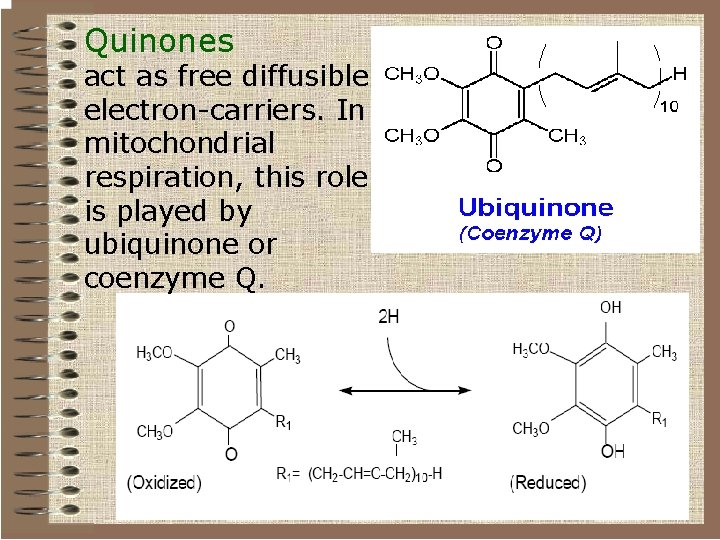
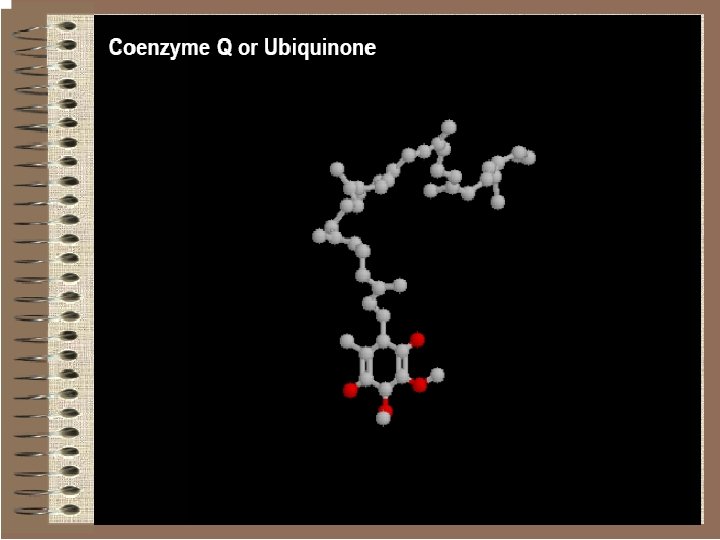
Quinones act as free diffusible electron-carriers. In mitochondrial respiration, this role is played by ubiquinone or coenzyme Q.



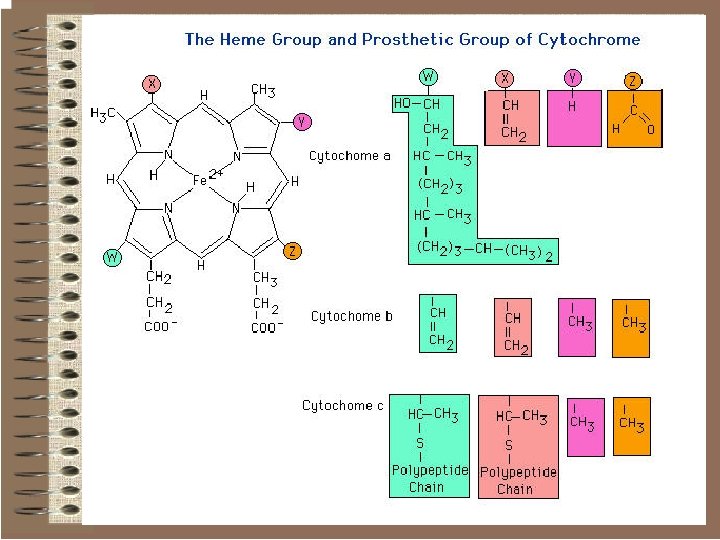
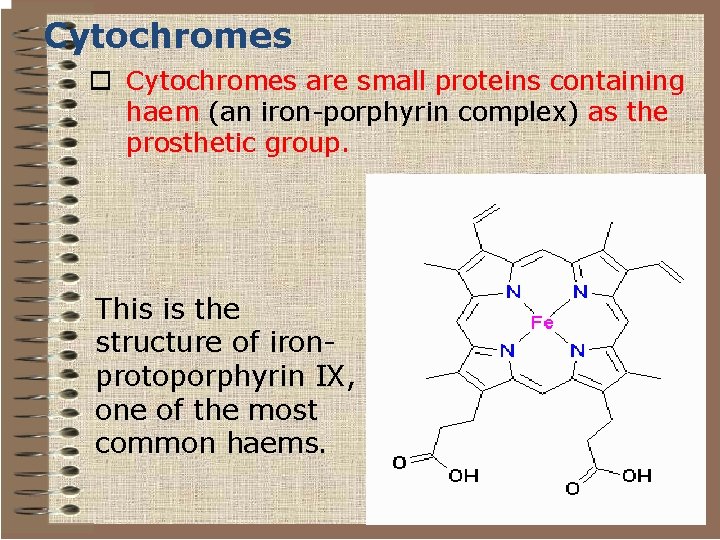
Cytochromes o Cytochromes are small proteins containing haem (an iron-porphyrin complex) as the prosthetic group. This is the structure of ironprotoporphyrin IX, one of the most common haems.

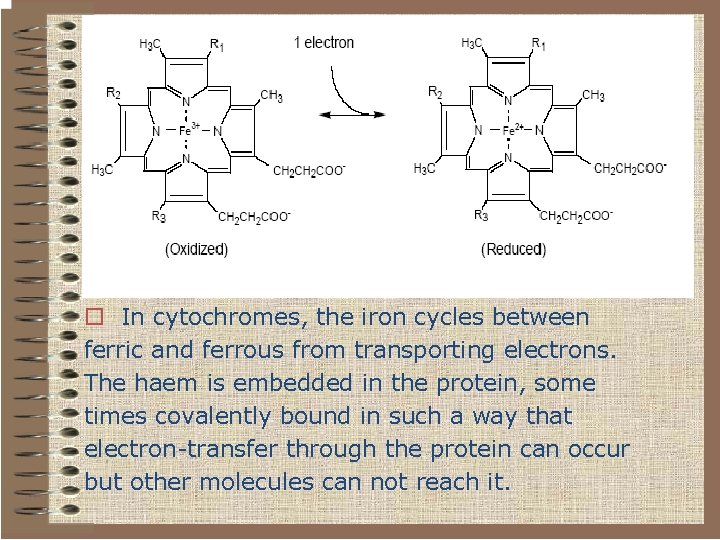
o In cytochromes, the iron cycles between ferric and ferrous from transporting electrons. The haem is embedded in the protein, some times covalently bound in such a way that electron-transfer through the protein can occur but other molecules can not reach it.


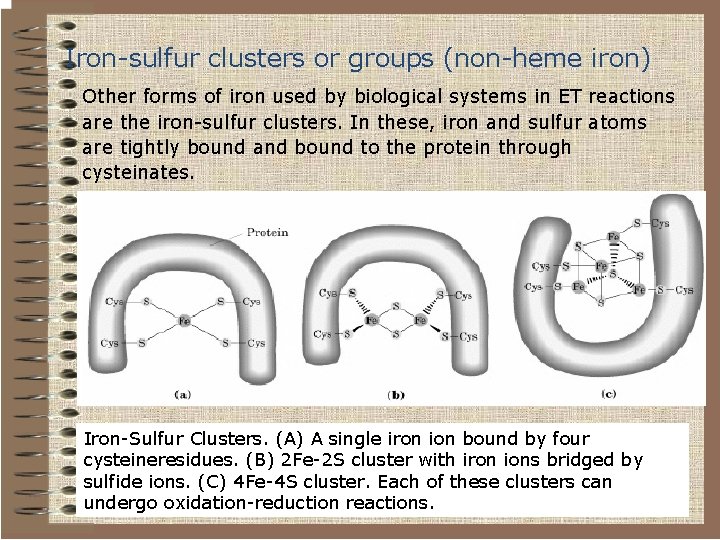
Iron-sulfur clusters or groups (non-heme iron) Other forms of iron used by biological systems in ET reactions are the iron-sulfur clusters. In these, iron and sulfur atoms are tightly bound and bound to the protein through cysteinates. Iron-Sulfur Clusters. (A) A single iron ion bound by four cysteineresidues. (B) 2 Fe-2 S cluster with iron ions bridged by sulfide ions. (C) 4 Fe-4 S cluster. Each of these clusters can undergo oxidation-reduction reactions.

Mitochondrial Electron Transport Organization 1. Most are organized into large multisubunit integral membrane protein complexes or coupling sites. • Complex I: NADH Dehydrogenase (MW = 750, 000) Coupling Site 1 • Complex II: Succinate Dehydrogenase– membrane bound enzyme which catalyzes the oxidation of succinate in the TCA-Cycle producing FADH 2 which-then reduces Ubiquinone. • Complex III: Cytochrome c Reductase (Cytochrome bc 1 complex) (MW = 280, 000). Coupling Site 2 • Complex IV: Cytochrome c Oxidase (Cytochrome aa 3) (MW= 200, 000) Coupling Site 3 -This reaction accounts for more than 90% of all the. O 2 consumed by living organisms.

• complex V, or ATP synthetase 2. Small Mobile Electron Carriers: • Ubiquinone /Ubiquinol-small hydrophobic electron carriers which shuttle electrons between the large complexes and back and forth across the lipid bilayer. • Cytochrome c– is a small water soluble protein which is a mobile electron carrier and carries electrons between cytochrome bc 1 complex and cytochrome c oxidase

Complex I

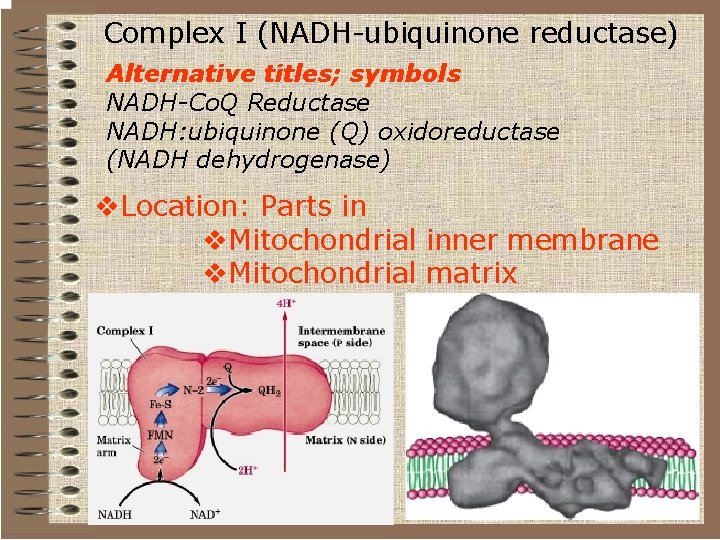
Complex I (NADH-ubiquinone reductase) Alternative titles; symbols NADH-Co. Q Reductase NADH: ubiquinone (Q) oxidoreductase (NADH dehydrogenase) v. Location: Parts in v. Mitochondrial inner membrane v. Mitochondrial matrix

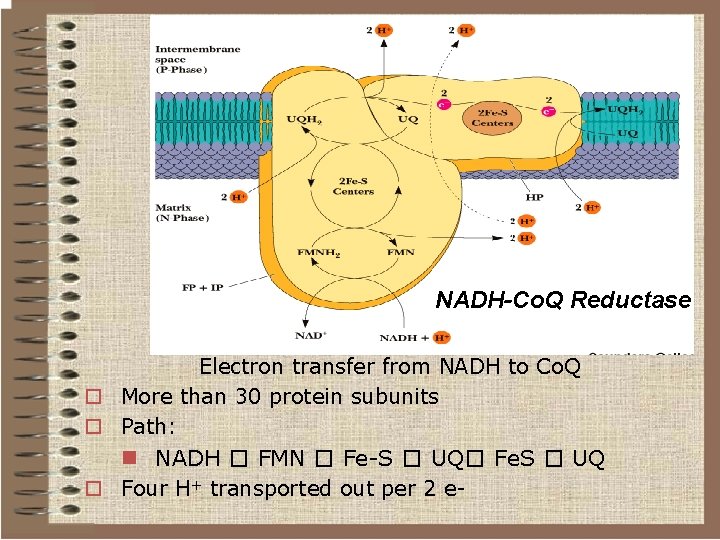
NADH-Co. Q Reductase Electron transfer from NADH to Co. Q o More than 30 protein subunits o Path: n NADH � FMN � Fe-S � UQ� Fe. S � UQ o Four H+ transported out per 2 e-

Complex I v. The first step in the ETC of MOP - v. The high-potential electrons of NADH enter the respiratory chain at NADH-Q (ubiquinone) Oxidoreductase (NADH dehydrogenase) Complex I • A multimeric assembly of 7 mitochondrial-encoded subunits (ND subunits) and at least 36 nuclearencoded subunits • Complex I Composition ØLargest respiratory chain complex Ø 46 protein assembly Ø 7 Mitochondrial encoded proteins Ø 39 nuclear encoded proteins

Ø Major subunits: Flavoprotein; Iron-sulfur protein; Hydrophobic fraction ØSubunits involved in Complex I structure o NDUFAB 1 (SDAP) : Carrier of fatty acid chain o NDUFA 1 (MWFE) : Primarily expressed in heart & skeletal muscle Ø Subunits involved in regulation of Complex I activity o NDUFS 4 (AQDQ) o Functions: Increased Complex I activity with phosphorylation

Complex I q. Associations (Respirasome) • Supercomplex of I, III, IV • Stabilizes structure of Complex I • Stoichiometry of I: III: IV = 1: 2: 4 • Deficiency in other Complexes may produce loss of Complex I function
 Reducing equivalents
Reducing equivalents Reducing equivalents
Reducing equivalents Electron carriers
Electron carriers Dhirubhaism
Dhirubhaism Reducing vs non reducing sugar
Reducing vs non reducing sugar Reducing sugar and non reducing sugar
Reducing sugar and non reducing sugar Glucose versus glycogen
Glucose versus glycogen Fehling's test
Fehling's test Bulk reducing product definition
Bulk reducing product definition Why according to robin and jay are people funny
Why according to robin and jay are people funny Think family ni
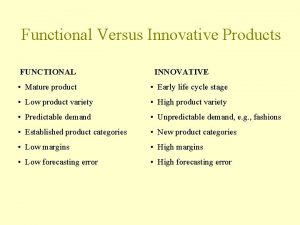
Think family ni Functional and innovative products examples
Functional and innovative products examples Pepsi 4ps marketing mix
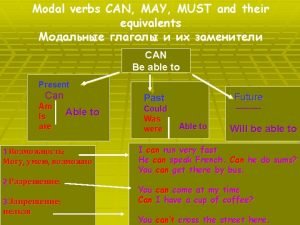
Pepsi 4ps marketing mix Modal verbs equivalents
Modal verbs equivalents Cash equivalents examples
Cash equivalents examples Adamn eve
Adamn eve Doctrine of equivalents test
Doctrine of equivalents test Match the dog idioms with their russian equivalents
Match the dog idioms with their russian equivalents Measurements equivalents and adjustments
Measurements equivalents and adjustments Nombre mixt
Nombre mixt Greek gods and roman equivalents
Greek gods and roman equivalents Apothecary conversion chart
Apothecary conversion chart The notion of equivalence in translation
The notion of equivalence in translation Ssri comparison chart side effects
Ssri comparison chart side effects Grade boundaries 2021
Grade boundaries 2021 Equivalent of must
Equivalent of must What is tablespoon abbreviation
What is tablespoon abbreviation The modal verb must
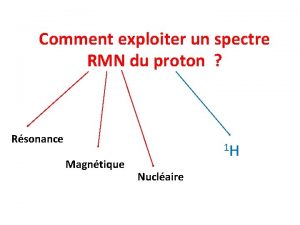
The modal verb must Spectre rmn ethanol
Spectre rmn ethanol Tablespoon abbreviation
Tablespoon abbreviation Repeat these words after your teacher
Repeat these words after your teacher Greek gods and roman equivalents
Greek gods and roman equivalents Name the two parts of a proverb
Name the two parts of a proverb Standard potential table
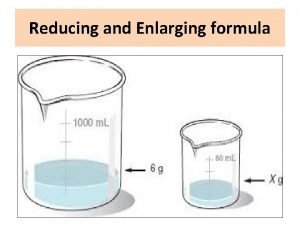
Standard potential table Reducing and enlarging formulas pharmacy examples
Reducing and enlarging formulas pharmacy examples Is ethanol bulk gaining or reducing
Is ethanol bulk gaining or reducing Adjective clause markers
Adjective clause markers Glucose standard curve
Glucose standard curve Structure of cellobiose
Structure of cellobiose Reducing ratios answer key
Reducing ratios answer key Redox reaction in alkaline medium
Redox reaction in alkaline medium Aldotriose
Aldotriose Why is sucrose not a reducing sugar
Why is sucrose not a reducing sugar Powerful oxidising agent
Powerful oxidising agent Reducing sugar in stool
Reducing sugar in stool Reducing and enlarging formula
Reducing and enlarging formula Adverb clause
Adverb clause Changing adjective clause to adjective phrase
Changing adjective clause to adjective phrase Bulk reducing industry ap human geography
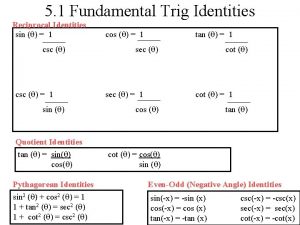
Bulk reducing industry ap human geography Sum and difference identitites
Sum and difference identitites Reducing substance in stool تحليل
Reducing substance in stool تحليل What works and doesn't in reducing recidivism download
What works and doesn't in reducing recidivism download Oxidizing agent examples
Oxidizing agent examples Introduction of gas welding
Introduction of gas welding Fehling's solution risk assessment
Fehling's solution risk assessment Reducing project duration
Reducing project duration Tan 15
Tan 15 Reducing balance method
Reducing balance method A key to reducing lot size without increasing costs is to
A key to reducing lot size without increasing costs is to Food tests
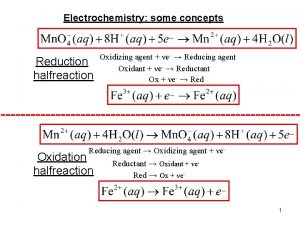
Food tests Reduction vs oxidation
Reduction vs oxidation Trehalose a reducing sugar
Trehalose a reducing sugar Reducing rational expressions to lowest terms
Reducing rational expressions to lowest terms What the
What the Reducing and enlarging formulas pdf
Reducing and enlarging formulas pdf What is the reducing end of a sugar
What is the reducing end of a sugar What is the development gap in jamaica
What is the development gap in jamaica Reducing project duration in project management
Reducing project duration in project management Map reducing in cloud computing
Map reducing in cloud computing Reducing rational expressions to lowest terms
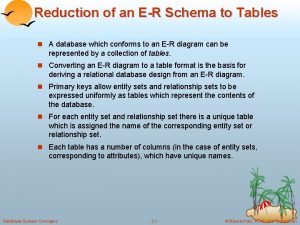
Reducing rational expressions to lowest terms Reducing er diagram to tables
Reducing er diagram to tables Techniques for reducing cache misses
Techniques for reducing cache misses Sulfate reducing bacteria
Sulfate reducing bacteria Identify oxidizing and reducing agents practice
Identify oxidizing and reducing agents practice Reducing balance method
Reducing balance method Bulk reducing examples
Bulk reducing examples Food test experiment
Food test experiment Noun clause to infinitive
Noun clause to infinitive Hemiacetal group in maltose
Hemiacetal group in maltose Principle of benedict test
Principle of benedict test Afterload reducing drugs
Afterload reducing drugs Adverbial clauses reduction
Adverbial clauses reduction Reducing and enlarging formulas pdf
Reducing and enlarging formulas pdf Techniques for reducing cache misses
Techniques for reducing cache misses Chapter 20 worksheet redox
Chapter 20 worksheet redox Critical word first and early restart
Critical word first and early restart Reducing agent
Reducing agent Reducing end of glycogen
Reducing end of glycogen Adverbial phrases exercises
Adverbial phrases exercises Relative strengths of oxidizing and reducing agents
Relative strengths of oxidizing and reducing agents Types of scale drawing
Types of scale drawing Differentiate between oxidizing and reducing agents
Differentiate between oxidizing and reducing agents Usually
Usually How often have you
How often have you Ini extension refers usually to what kind of file
Ini extension refers usually to what kind of file Private markets usually fail to provide lighthouses because
Private markets usually fail to provide lighthouses because King henry mnemonic
King henry mnemonic Speeches on questions of fact are usually organized
Speeches on questions of fact are usually organized They are usually wearing coats in winter
They are usually wearing coats in winter Yoga and reza ... do their homework last night. *
Yoga and reza ... do their homework last night. * An uncontrolled railroad crossing
An uncontrolled railroad crossing Mignonne masculine
Mignonne masculine Businesses often call their stores and warehouses
Businesses often call their stores and warehouses Wat is de present simple
Wat is de present simple Language
Language Chorus of
Chorus of