Waves Photons the EM Spectrum Astronomers obtain information

Waves, Photons & the EM Spectrum

Astronomers obtain information about the universe mainly via analysis of electromagnetic (em) radiation: visible light radio waves x-rays infrared radiation and so on. . . · EM radiation sometimes behaves like waves, sometimes like particles!

Waves
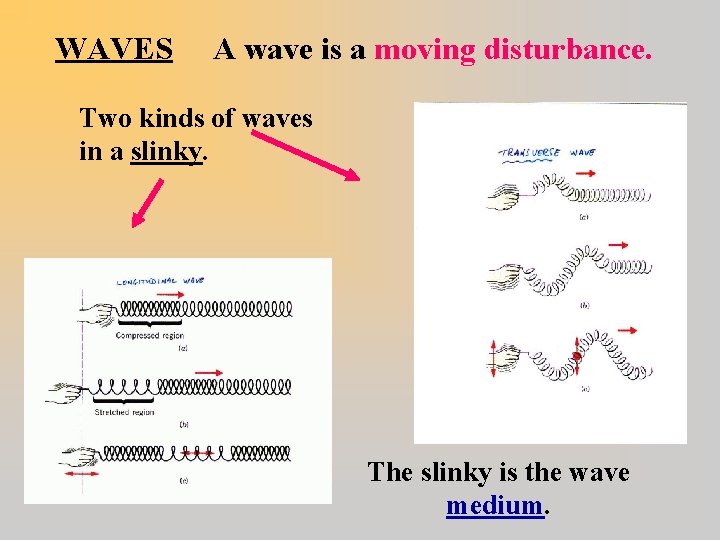
WAVES A wave is a moving disturbance. Two kinds of waves in a slinky. The slinky is the wave medium.
![[Wave animations] [Wave animations]](http://slidetodoc.com/presentation_image_h2/55932aaa0eb97df4c7631f6671856149/image-5.jpg)
[Wave animations]
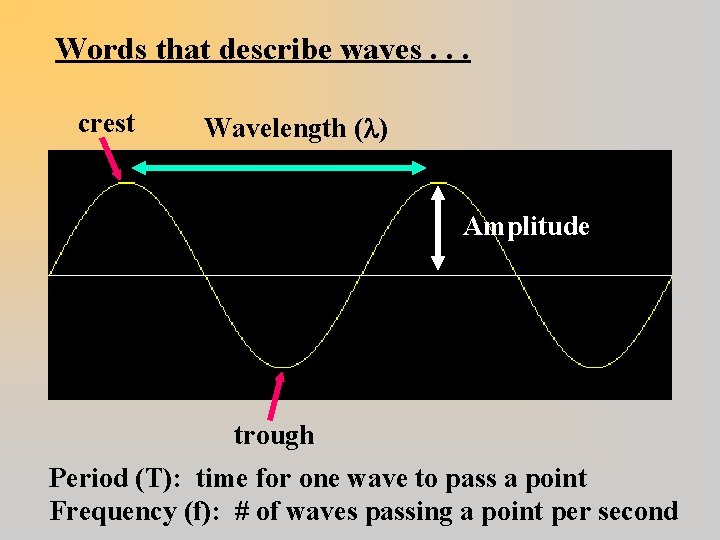
Words that describe waves. . . crest Wavelength ( ) Amplitude trough Period (T): time for one wave to pass a point Frequency (f): # of waves passing a point per second
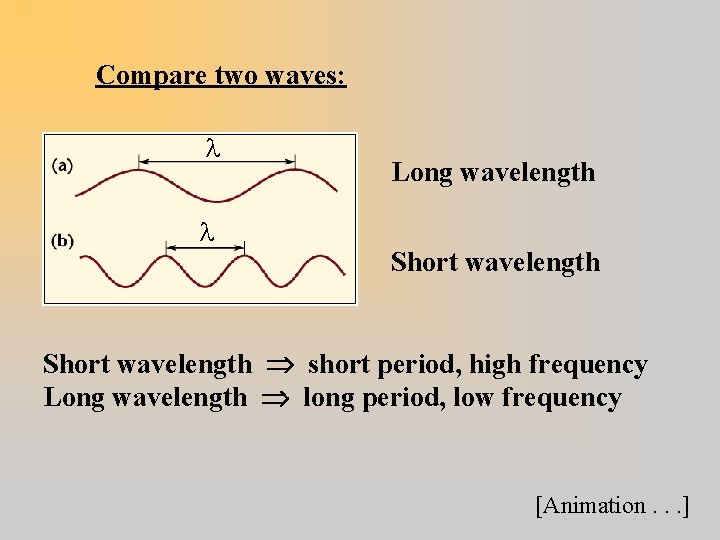
Compare two waves: Long wavelength Short wavelength short period, high frequency Long wavelength long period, low frequency [Animation. . . ]
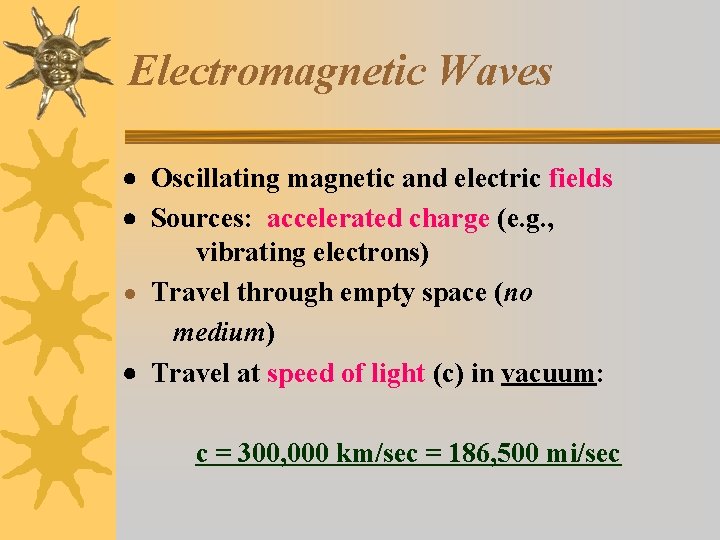
Electromagnetic Waves Oscillating magnetic and electric fields Sources: accelerated charge (e. g. , vibrating electrons) · Travel through empty space (no medium) Travel at speed of light (c) in vacuum: c = 300, 000 km/sec = 186, 500 mi/sec
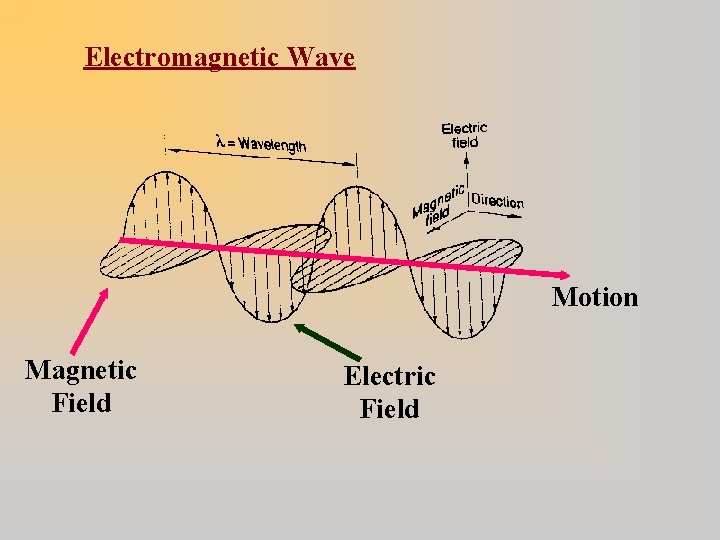
Electromagnetic Wave Motion Magnetic Field Electric Field
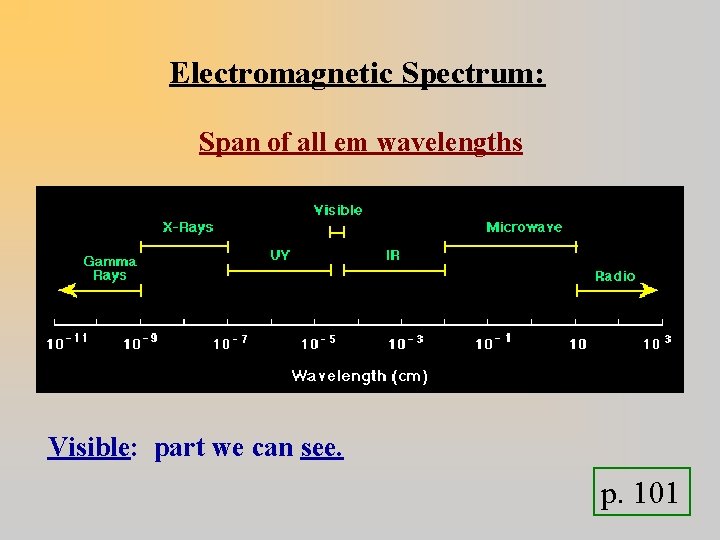
Electromagnetic Spectrum: Span of all em wavelengths Visible: part we can see. p. 101
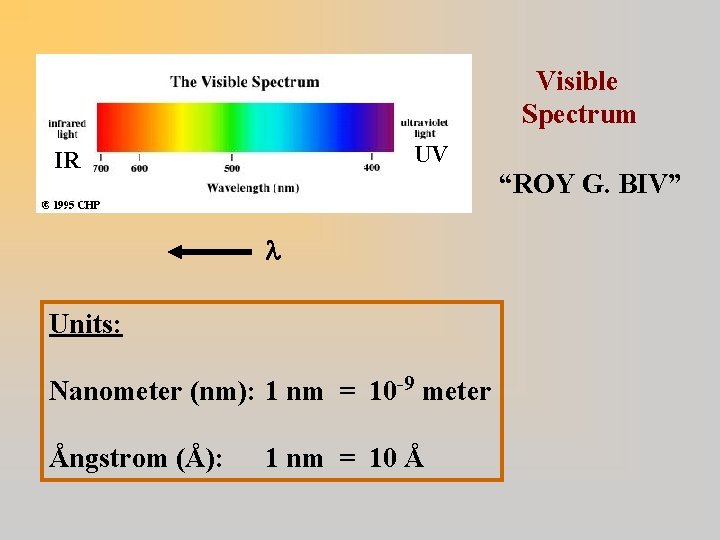
Visible Spectrum UV IR “ROY G. BIV” Units: Nanometer (nm): 1 nm = 10 -9 meter Ångstrom (Å): 1 nm = 10 Å

Photons ¬ 1900 – 1905: Max Planck & Albert Einstein find light sometimes behaves like particles: photons ¬ Photons carry energy (E): E Frequency (E f), or E 1/Wavelength (E 1/ )
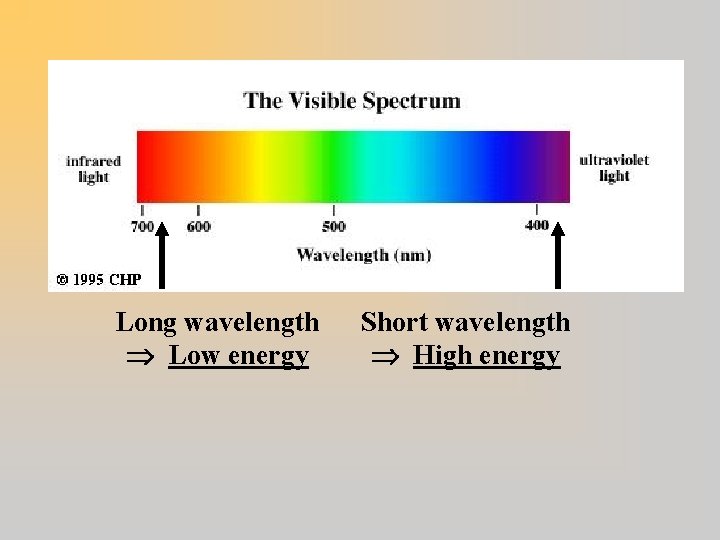
Long wavelength Low energy Short wavelength High energy
Interaction of Light & Matter 1. Emission 2. Absorption 3. Transmission 4. Reflection
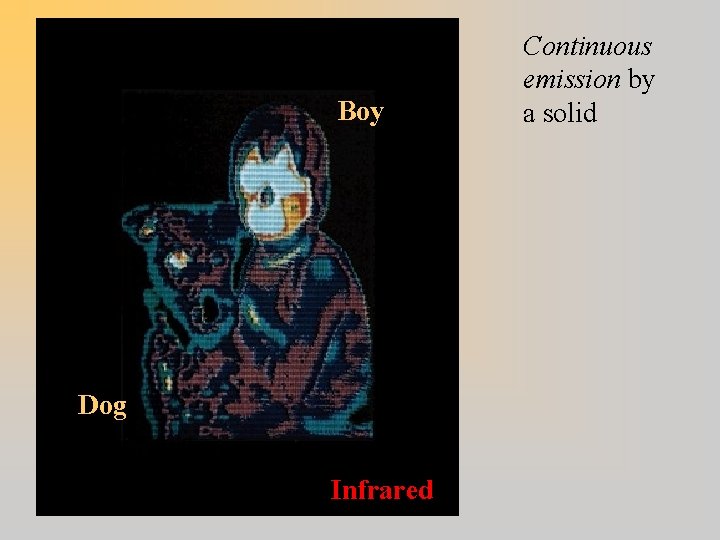
Boy Dog Infrared Continuous emission by a solid

‘Cool’ ‘Warm’ ‘Hot’
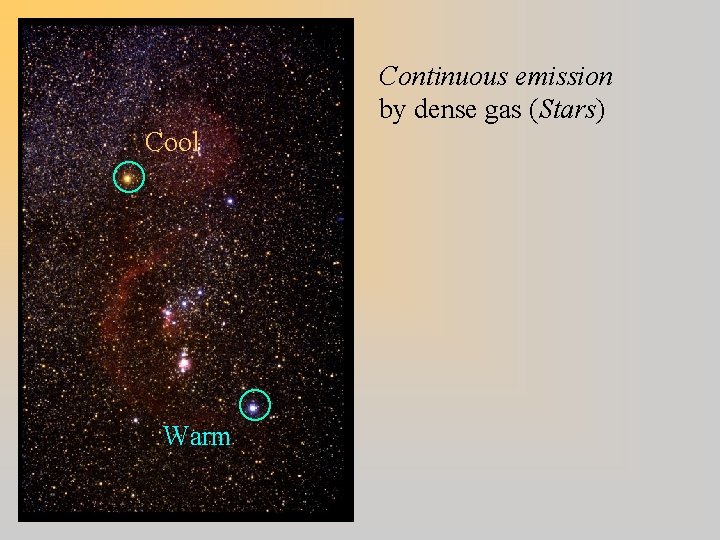
Continuous emission by dense gas (Stars) Cool Warm

Selective emission by a thin gas

Selective emission by a thin gas

white light Selective reflection & absorption by solids

selective reflection & absorption by solids & gases

Spectra I procured a triangular glass prism, to try therewith, the celebrated phenomena of colors. And for that purpose, having darkened my laboratory, and made a small hole in my window shade, to let in a convenient quantity of the sun’s light, I placed my prism at the entrance, that the light might be thereby refracted to the opposite wall. It was at first a very pleasing diversion to view the vivid and intense colors produced thereby. - Isaac Newton
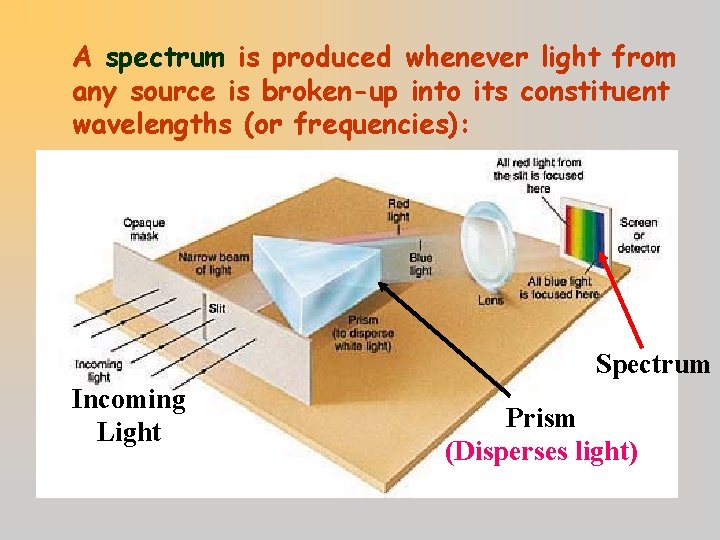
A spectrum is produced whenever light from any source is broken-up into its constituent wavelengths (or frequencies): Spectrum Incoming Light Prism (Disperses light)

Three Types of Spectra 1. Emission (Bright) Line Bright lines on a dark background 2. Absorption Line Dark lines on a bright background 3. Continuous band of colors
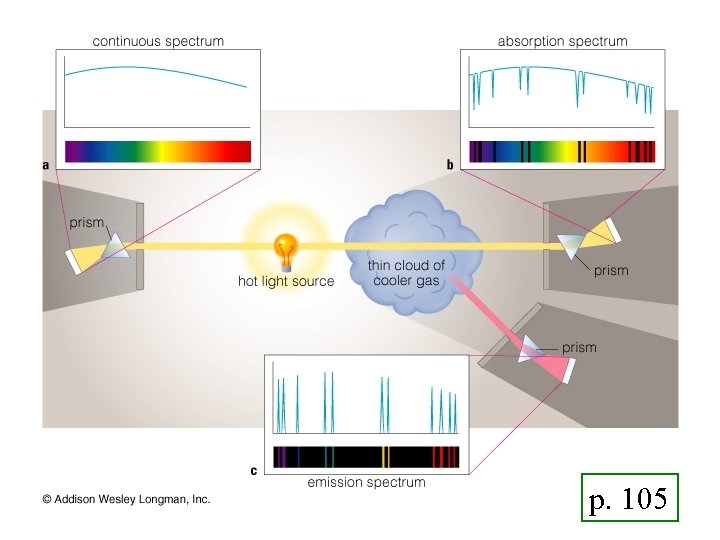
p. 105
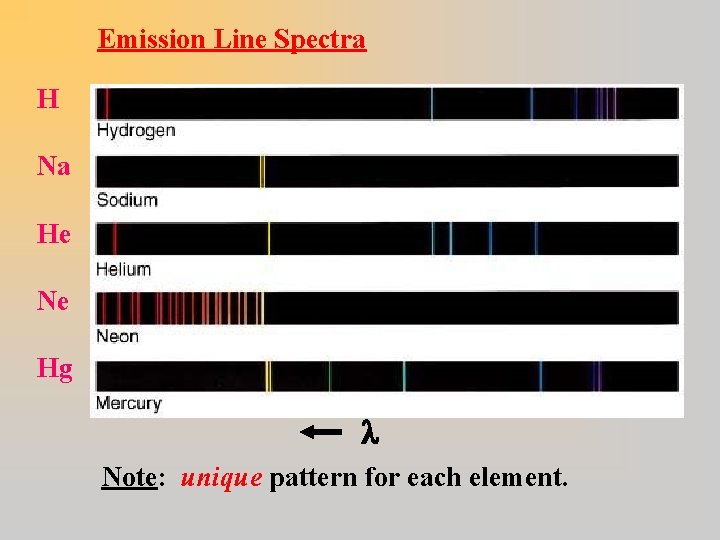
Emission Line Spectra H Na He Ne Hg Note: unique pattern for each element.
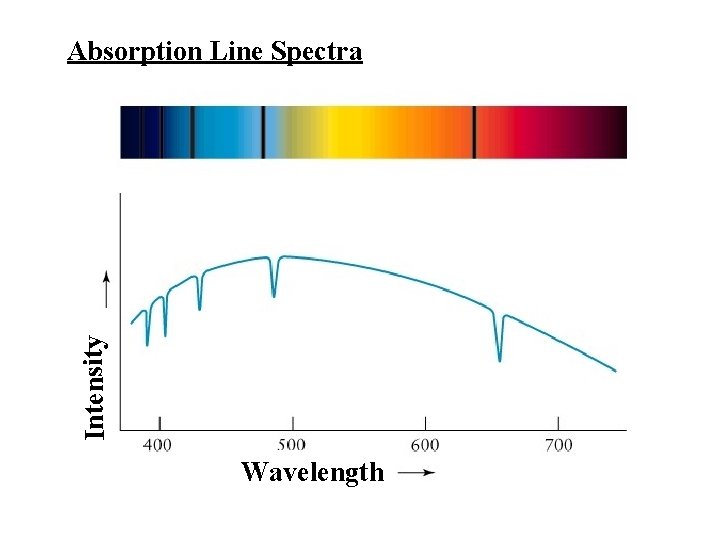
Intensity Absorption Line Spectra Wavelength
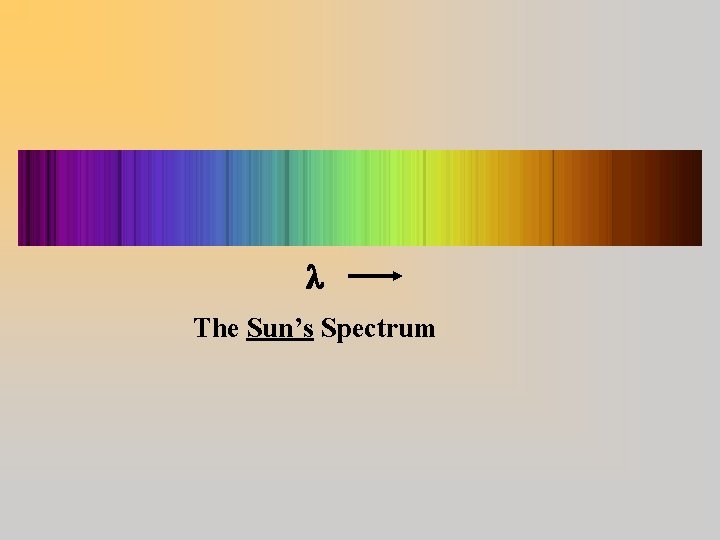
The Sun’s Spectrum
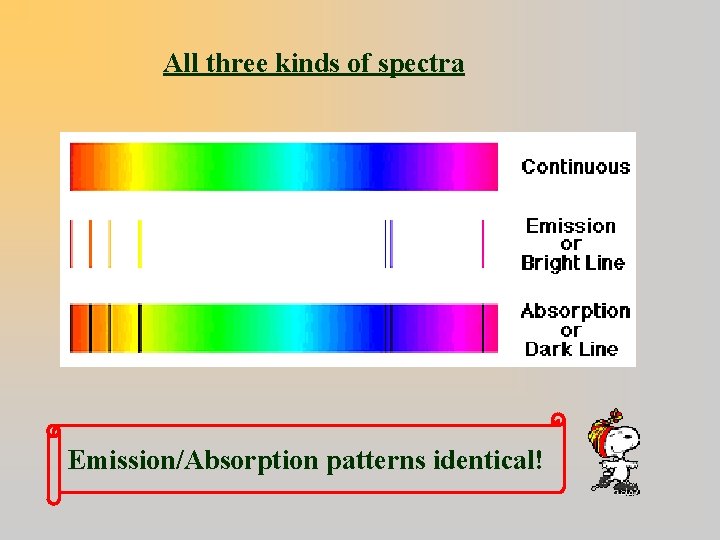
All three kinds of spectra Emission/Absorption patterns identical!
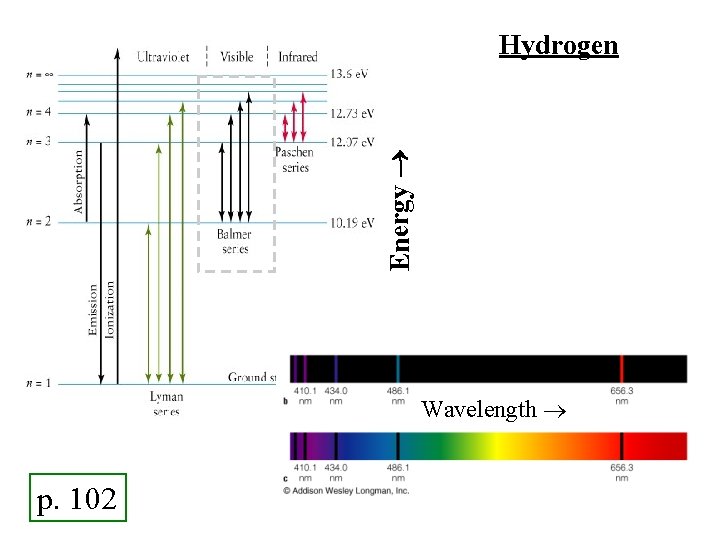
Energy Hydrogen Wavelength p. 102
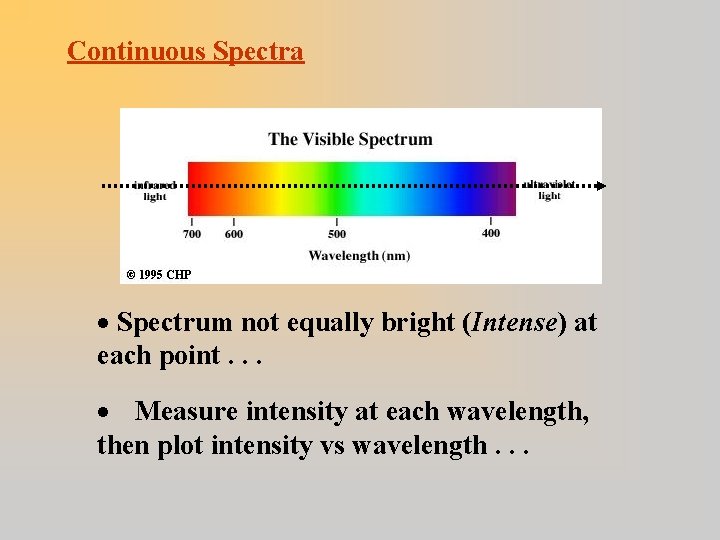
Continuous Spectra · Spectrum not equally bright (Intense) at each point. . . · Measure intensity at each wavelength, then plot intensity vs wavelength. . .
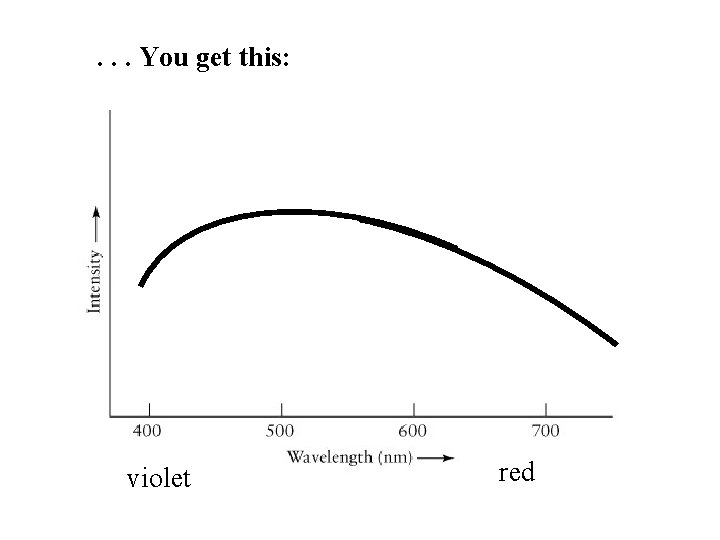
. . . You get this: violet red
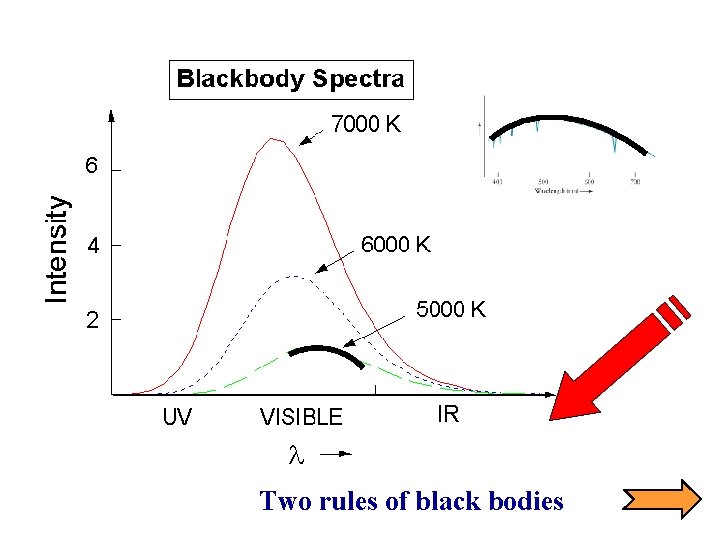
Two rules of black bodies
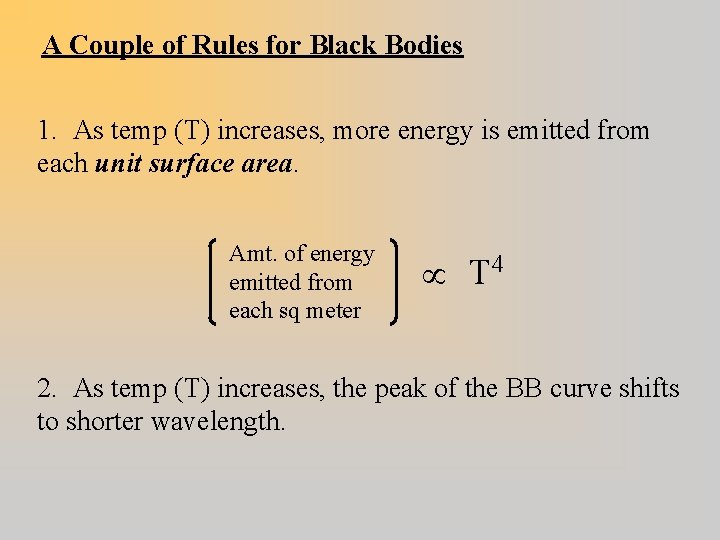
A Couple of Rules for Black Bodies 1. As temp (T) increases, more energy is emitted from each unit surface area. Amt. of energy emitted from each sq meter T 4 2. As temp (T) increases, the peak of the BB curve shifts to shorter wavelength.
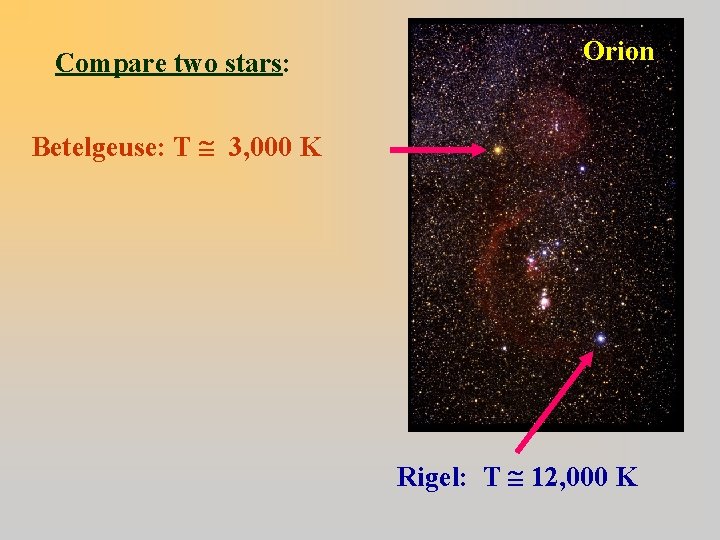
Compare two stars: Orion Betelgeuse: T 3, 000 K Rigel: T 12, 000 K
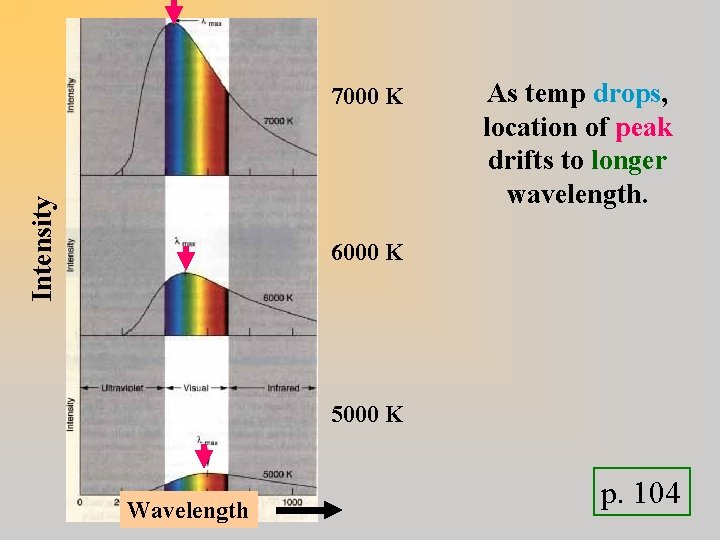
Intensity 7000 K As temp drops, location of peak drifts to longer wavelength. 6000 K 5000 K Wavelength p. 104
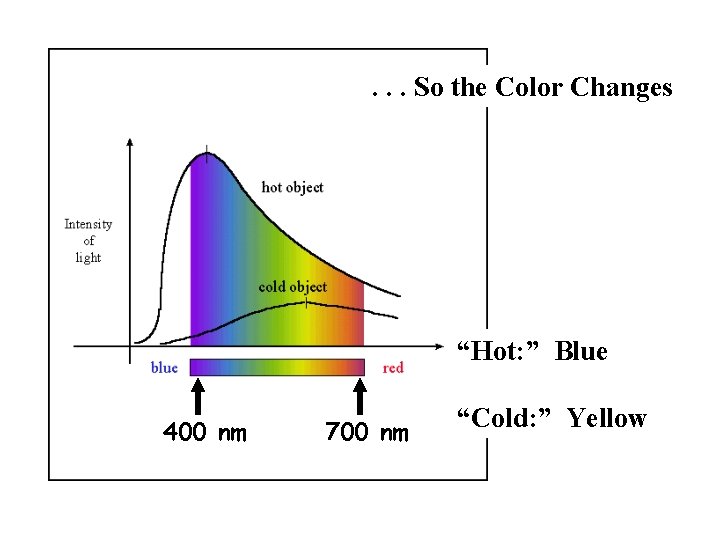
. . . So the Color Changes “Hot: ” Blue 400 nm 700 nm “Cold: ” Yellow
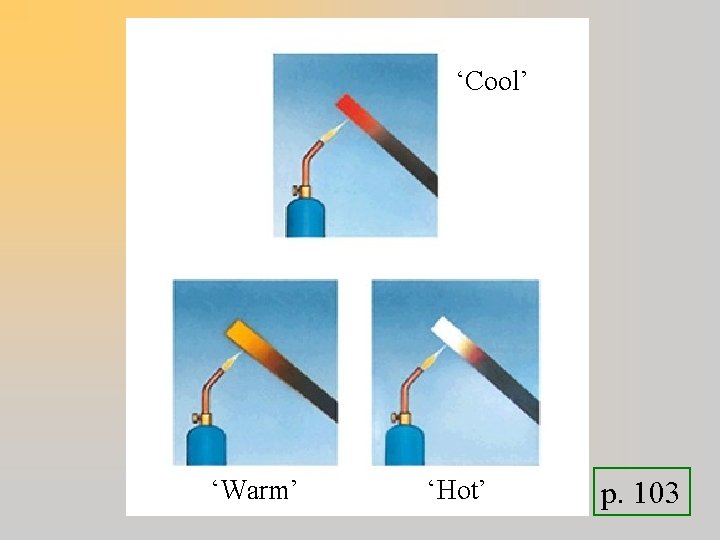
‘Cool’ ‘Warm’ ‘Hot’ p. 103
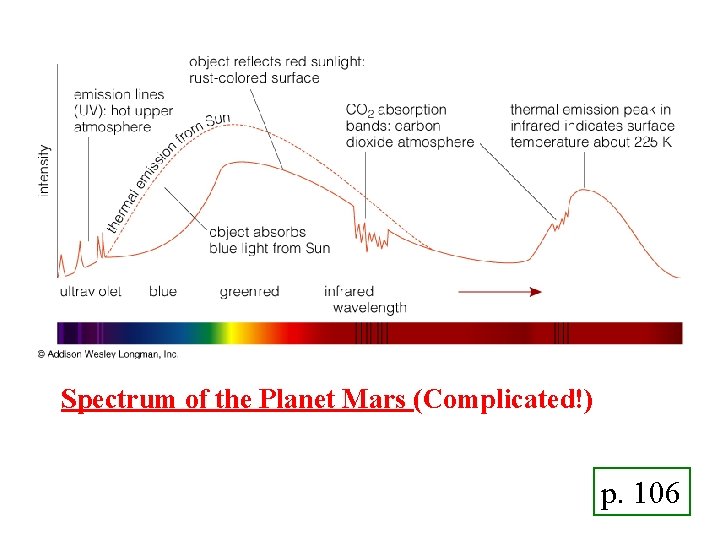
Spectrum of the Planet Mars (Complicated!) p. 106
The Doppler Effect: Change in observed wavelength and frequency of waves due to radial motion of source and/or observer.
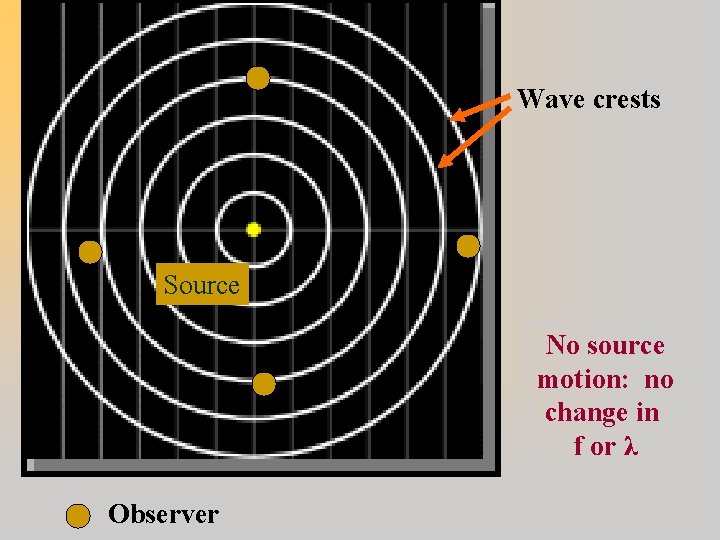
Wave crests Source No source motion: no change in f or λ Observer
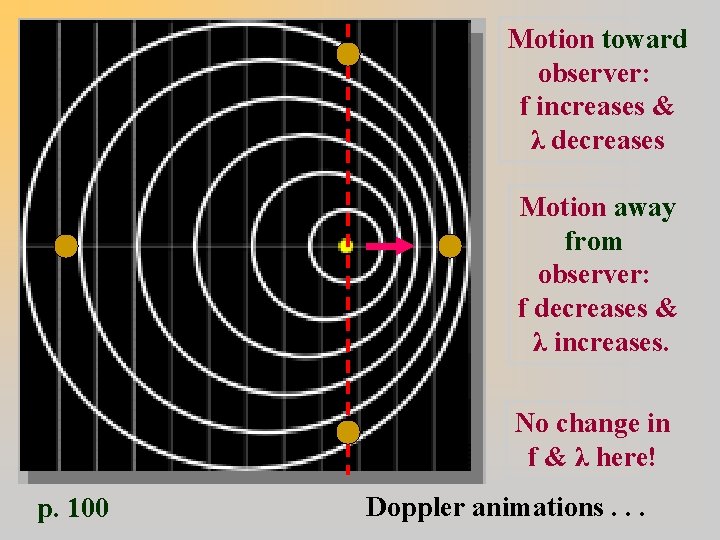
Motion toward observer: f increases & λ decreases Motion away from observer: f decreases & λ increases. No change in f & λ here! p. 100 Doppler animations. . .

Astronomically speaking. . . For a star moving toward/away from Earth. . .
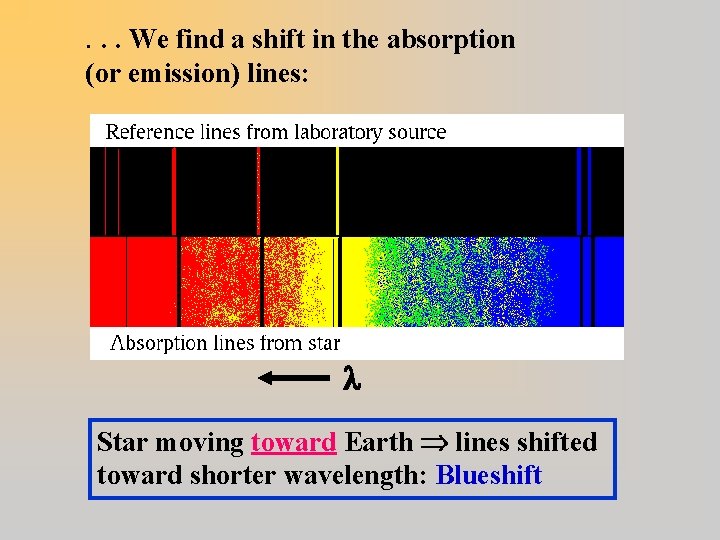
. . . We find a shift in the absorption (or emission) lines: Star moving toward Earth lines shifted toward shorter wavelength: Blueshift
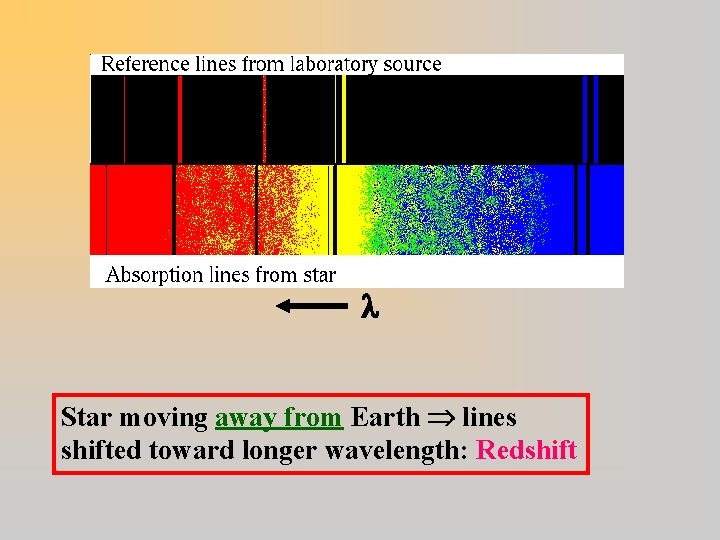
Star moving away from Earth lines shifted toward longer wavelength: Redshift
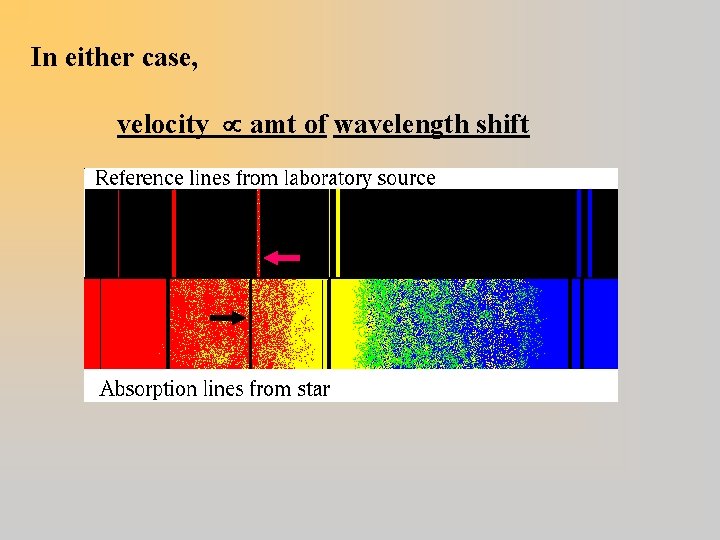
In either case, velocity amt of wavelength shift
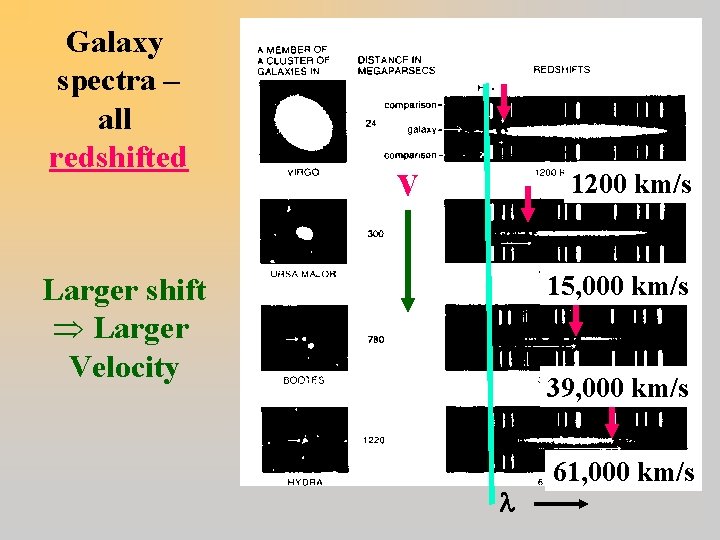
Galaxy spectra – all redshifted v 1200 km/s 15, 000 km/s Larger shift Þ Larger Velocity 39, 000 km/s 61, 000 km/s
- Slides: 47