Wavelength Distance between identical points on consecutive waves
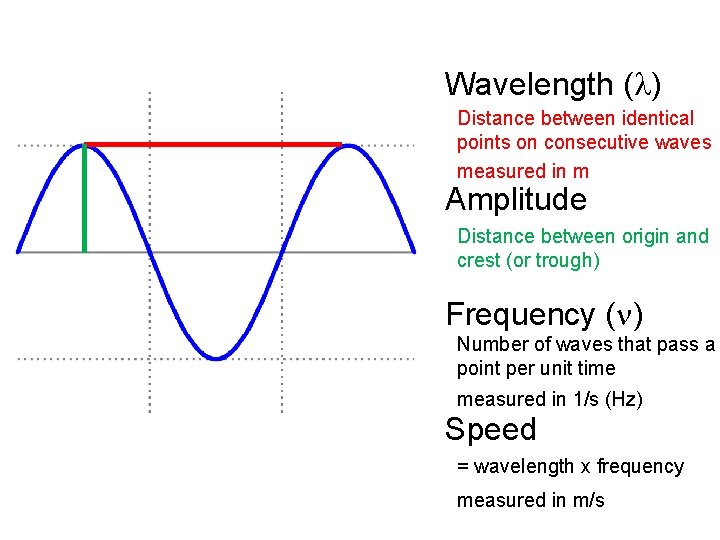
Wavelength ( ) Distance between identical points on consecutive waves measured in m Amplitude Distance between origin and crest (or trough) Frequency ( ) Number of waves that pass a point per unit time measured in 1/s (Hz) Speed = wavelength x frequency measured in m/s
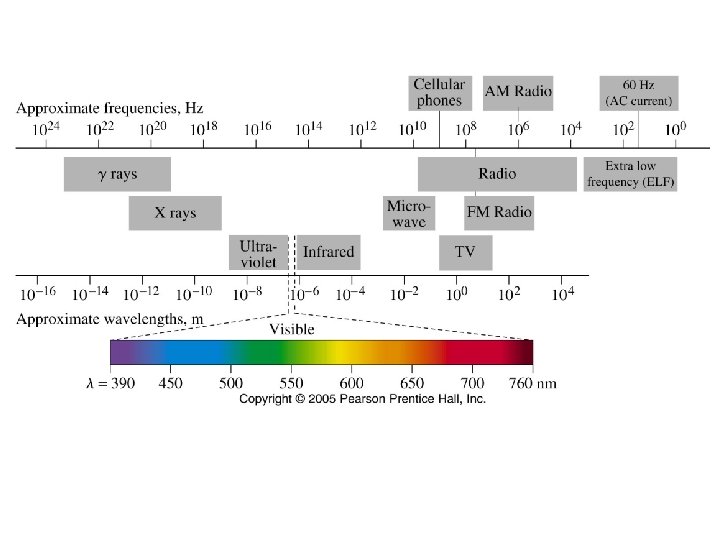
1900 – Max Planck • studying blackbody radiation • energy emitted/absorbed is quantized • E = h

1905 – Albert Einstein
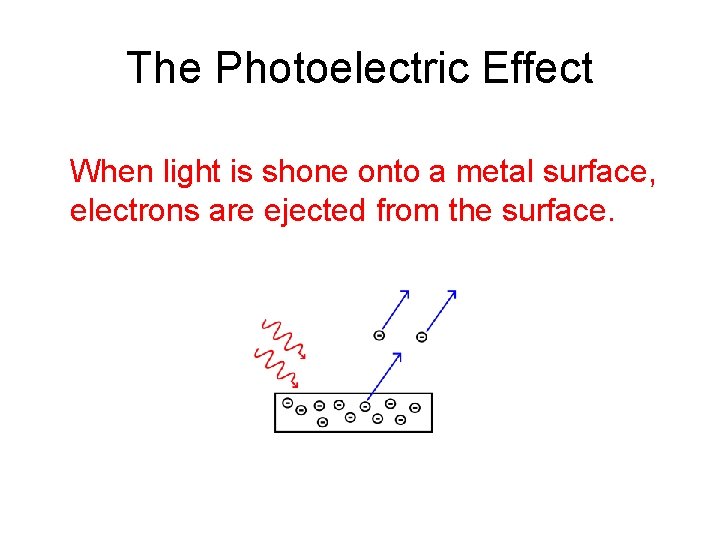
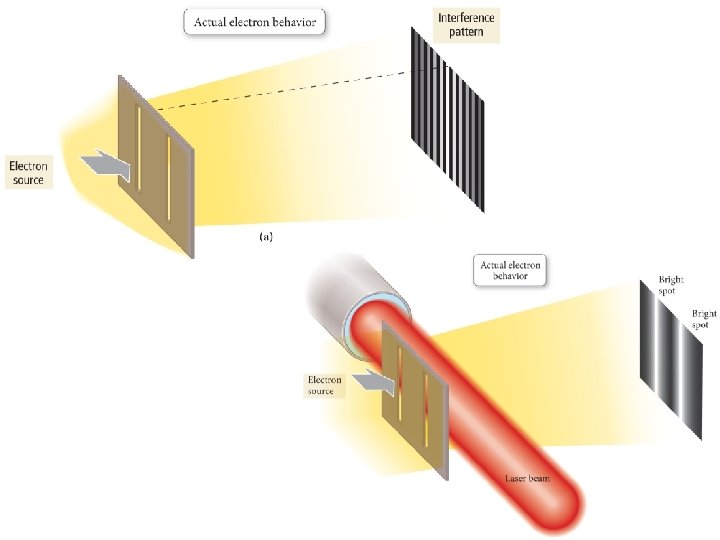
The Photoelectric Effect When light is shone onto a metal surface, electrons are ejected from the surface.
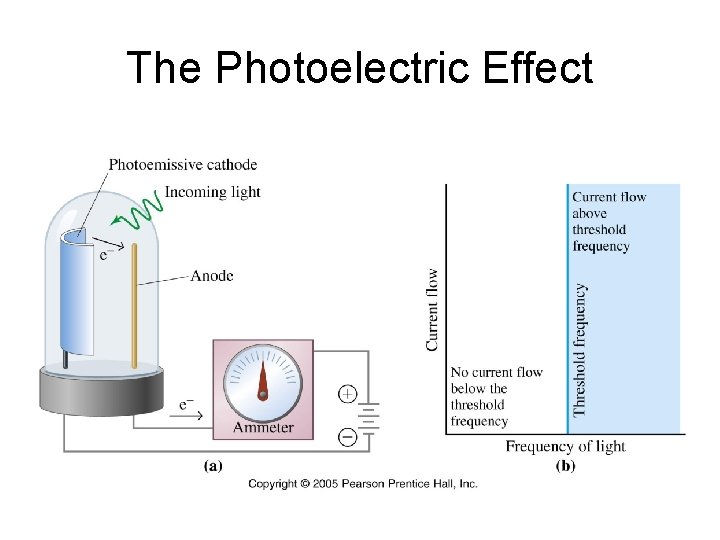
The Photoelectric Effect
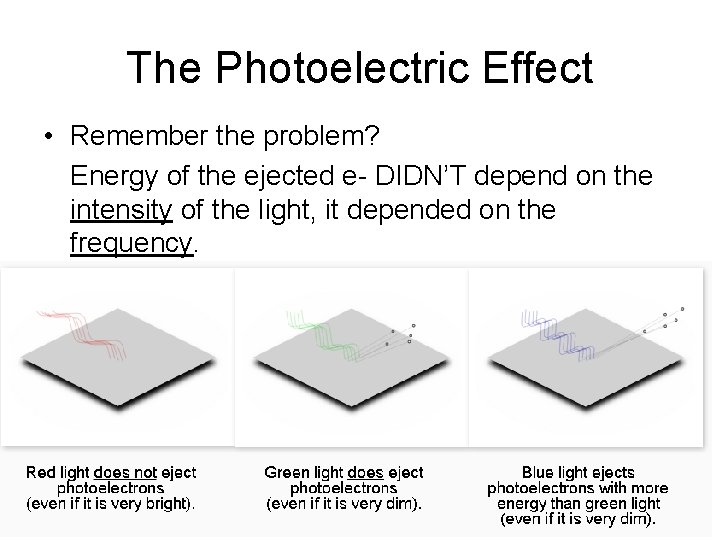
The Photoelectric Effect • Remember the problem? Energy of the ejected e- DIDN’T depend on the intensity of the light, it depended on the frequency.

1905 – Albert Einstein • studying the photoelectric effect • proposed that energy itself is quantized • energy exists as particles Can I get a Energy collective gasp from as PARTICLES!? ! the audience?

“The particle-wave duality of light” Light has particle-like properties as well as wave-like properties.
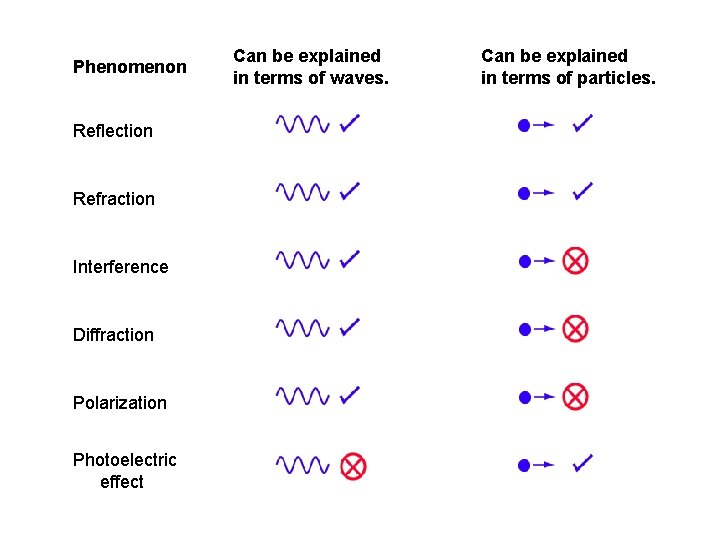
Phenomenon Reflection Refraction Interference Diffraction Polarization Photoelectric effect Can be explained in terms of waves. Can be explained in terms of particles.

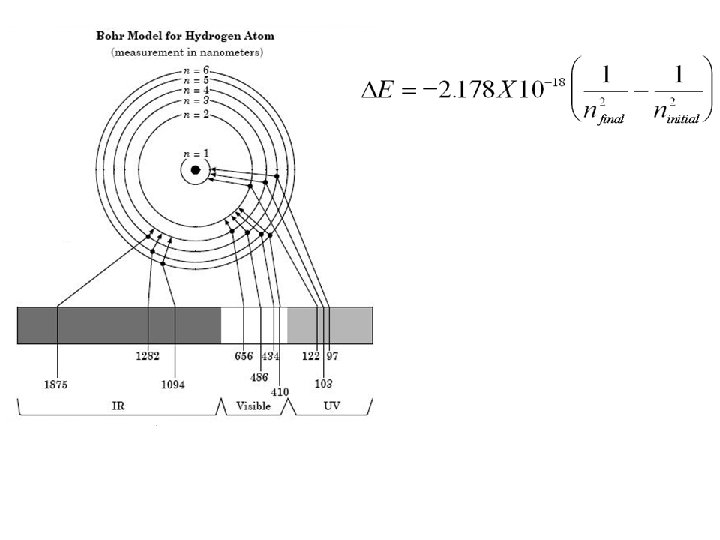
1913 – Niels Bohr Hydrogen Line Emission Spectrum

1913 – Niels Bohr • proposed that the energy of the electron in the H atom was quantized (electron can only have certain amounts • allowed of energy, energy not any levels foramount) the possible electron = ORBITS

1913 – Niels Bohr • The electron in a hydrogen atom is usually in the n = 1 energy level. This is called the ground state for that atom. • When energy is supplied, the electron can move into a higher energy level. The atom is then said to be in an excited state. • When the e- returns to its ground state, energy is released.

1924 – Louis de Broglie • proposed that electrons have wave properties
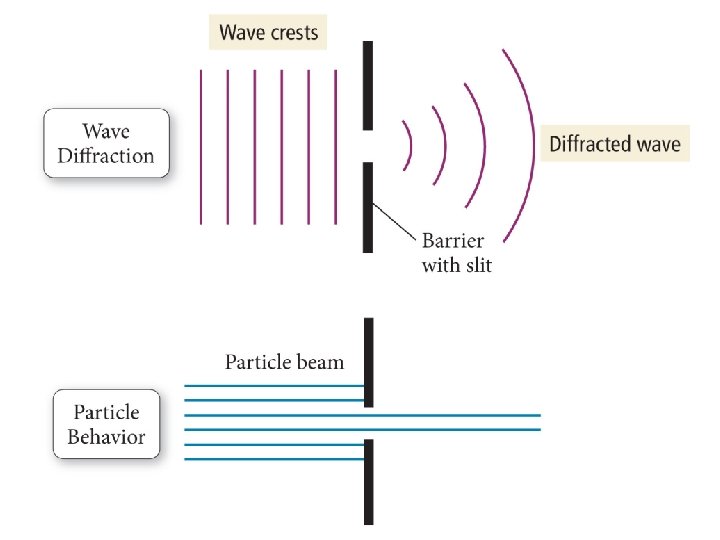
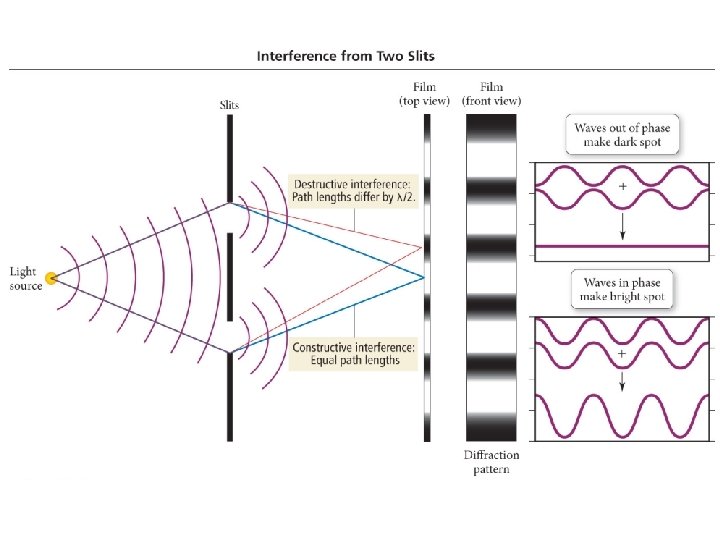
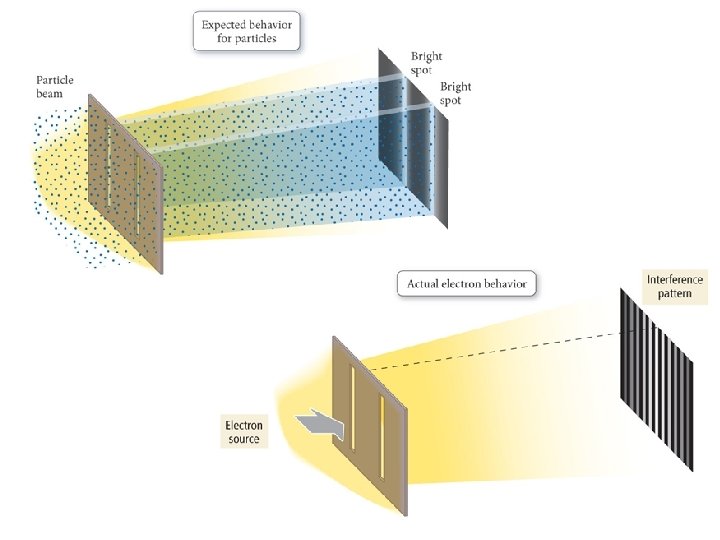
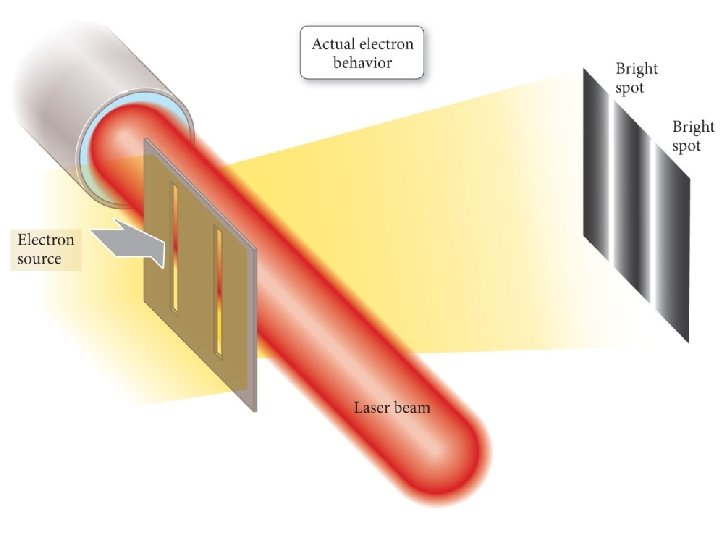
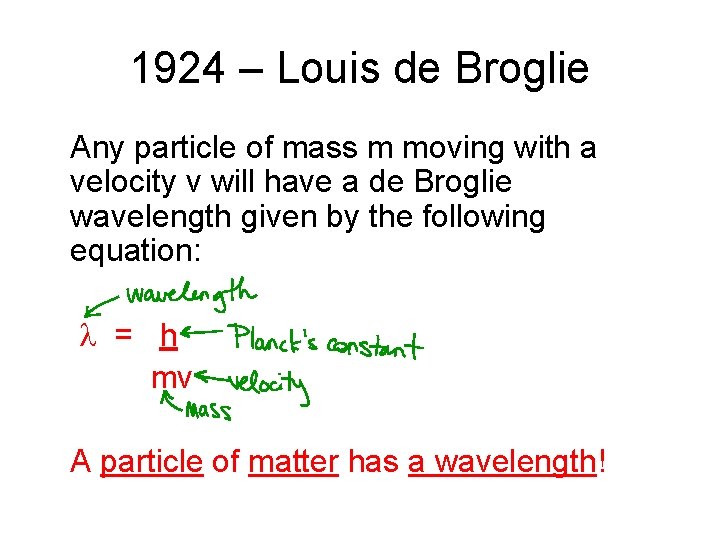
1924 – Louis de Broglie Any particle of mass m moving with a velocity v will have a de Broglie wavelength given by the following equation: = h mv A particle of matter has a wavelength!

1926 – Erwin Schrödinger
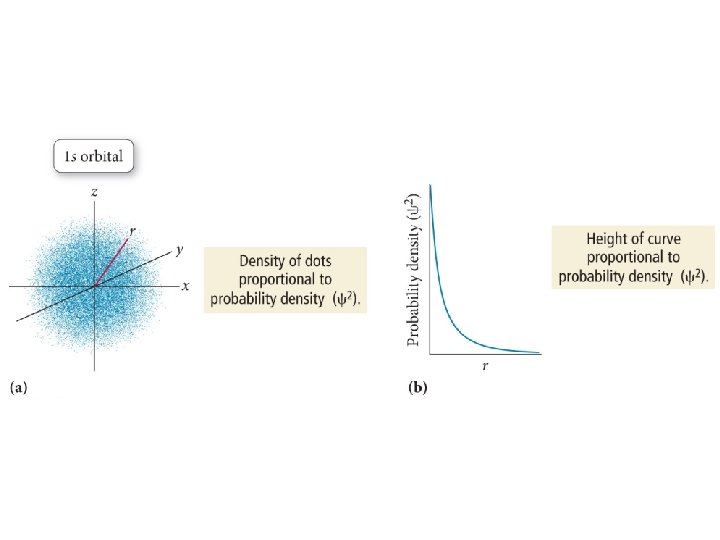
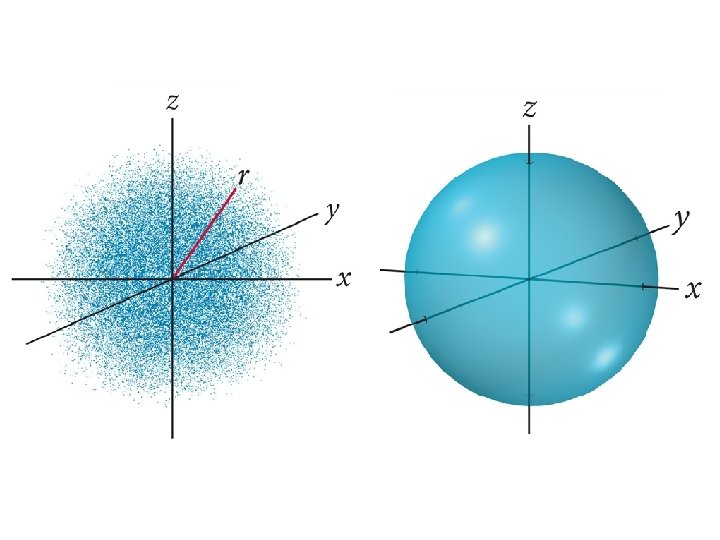
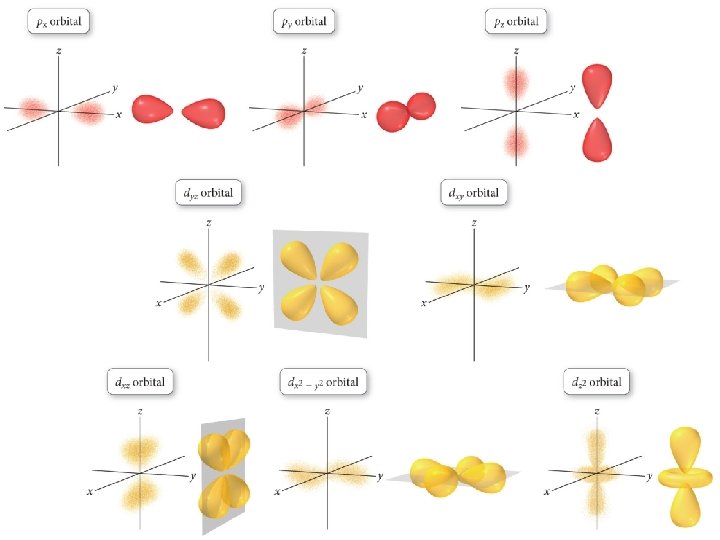
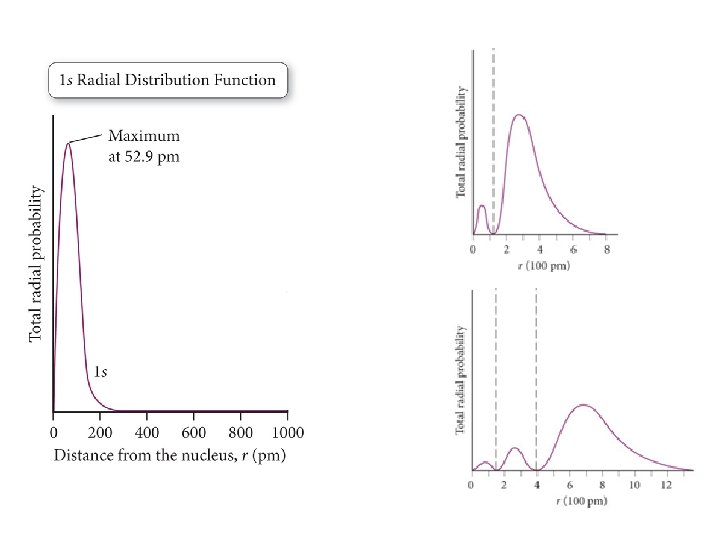
Order of Orbital Filling? 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p
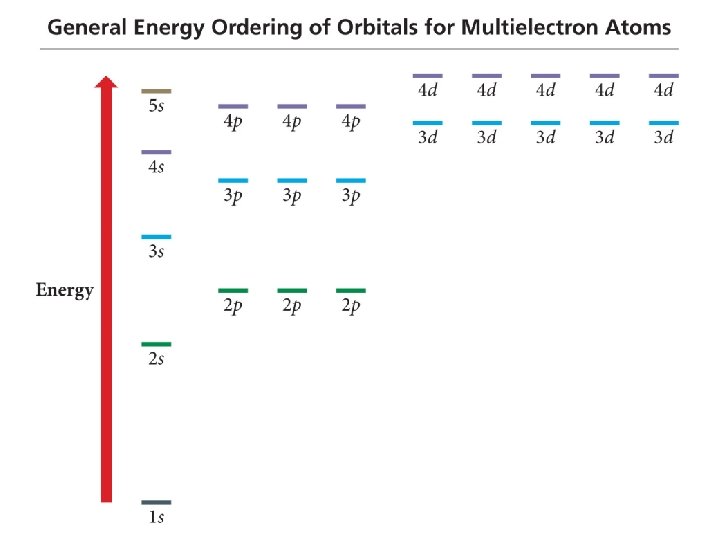
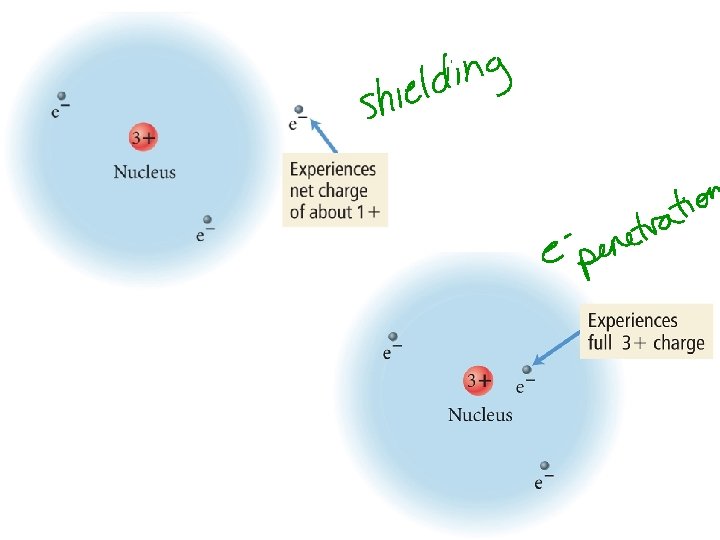
Order of Orbital Filling? 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d 5 p WHY? • Sublevel Energy Splitting – Coulomb’s Law – Nuclear Shielding – Electron Penetration • Spatial distribution of electrons in sublevels
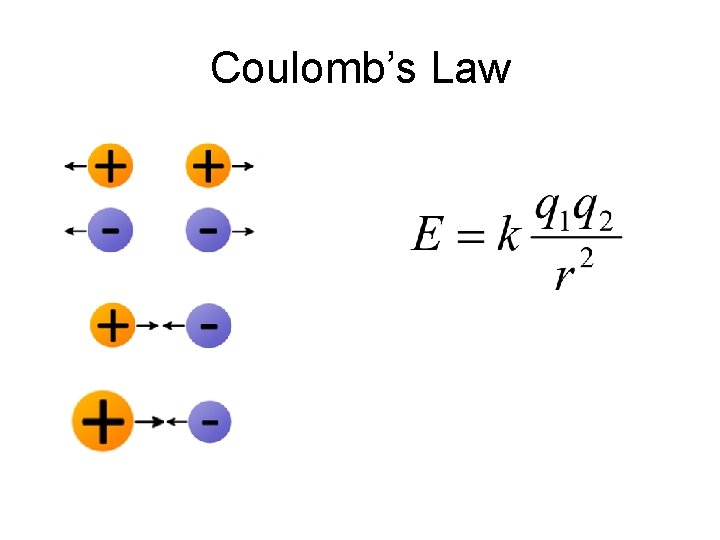
Coulomb’s Law
Shielding image from chemwiki. ucdavis. edu
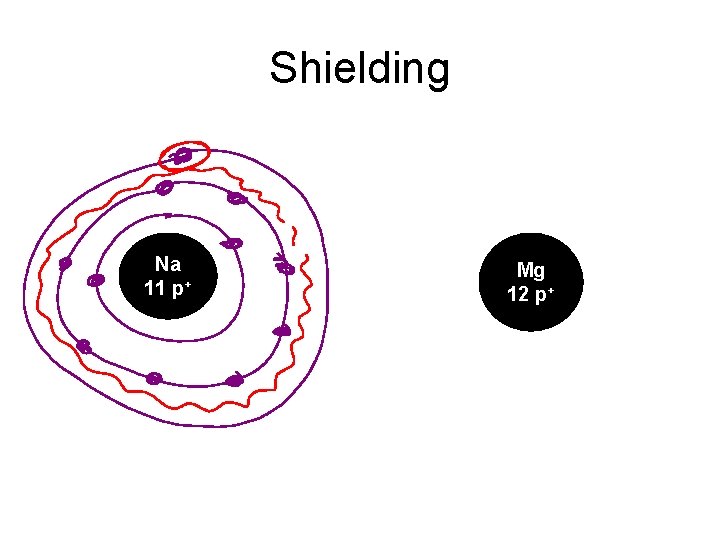
Shielding Na 11 p+ Mg 12 p+
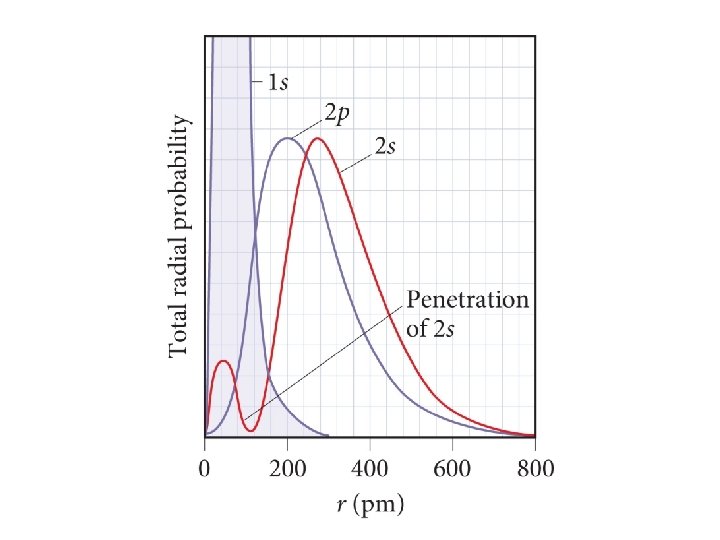
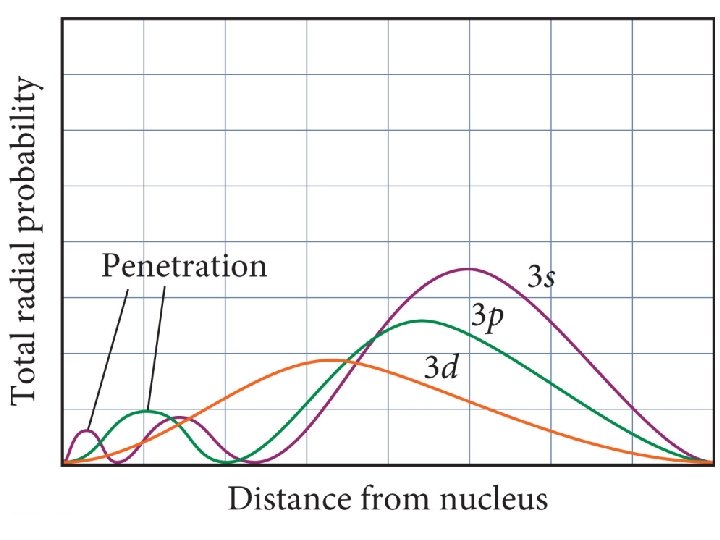
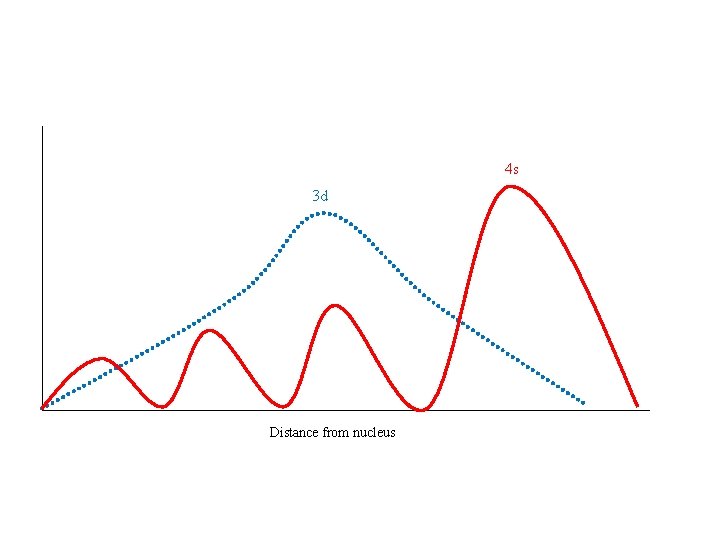
4 s 3 d Distance from nucleus

https: //www. youtube. com/watch? v=BMIv. Wz-7 Gm. U
- Slides: 36