Water Treatment Water Treatment Average household uses 300





















- Slides: 25

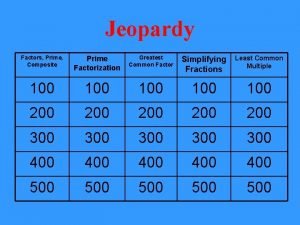
Water Treatment

Water Treatment • Average household uses 300 litres per day Criteria – Colourless – Odourless – Safe to drink (no active bacteria) – Fluorinated To ensure water is of an adequate standard for drinking, it must be treated

7 Stages Water from Lakes and Rivers Screening Flocculation Settlement Filtration Chlorination Fluoridation p. H Adjustment Water to homes and industry


Screening 1 st stage Flocculation 2 nd stage * Wire mesh * Removes large solids and floating debris like twigs, plastics etc * flocculating agent ( or flocculant) usually aluminium sulphate (alum) is added. * Makes smaller suspended solids coagulate or stick together in large clumps, so they are easier to remove at the next stage

Settlement 3 rd stage Filtration 4 th stage * Large tanks * Water goes in at the bottom and rises slowly to the top, at < 2 m/hr * Particles settle to the bottom * Over 90% of suspended solids removed at this stage * Large beds of sand * * Removes remaining susp solids Sand supported on a layer of gravel Sand cleaned regularly Water now clear but may contain harmful bacteria

Chlorination * Cl 2 gas is added 5 th stage * * Sterilises water Very small quantities 0. 2 – 0. 5 ppm Controlled carefully Monitored by bacteriological exam of the water Fluoridation * Na. F or H 2 Si. F 6 added 6 th stage (hexafluorosilicic acid) * Added by law as helps to reduce dental decay by strengthening the enamel * Small quantities ~ 1 ppm

p. H Adjustment 7 th stage • Optimum level is between 7 - 9 • Too Acidic * may cause damage to pipes * may be corrected by addition of Ca(OH)2 (lime)to raise the p. H * If very hard water, might be softened by addition of Na. CO 3 which is a base • Too Basic * may be corrected by addition of dilute H 2 SO 4 to lower the p. H Water is now ready for consumption and use

Surface Water Quality • Contaminants: – Suspended solids, soil (turbidity) – Pathogens (coliform indicator) – Color (decaying vegetation, algae) – Taste & odor – Other SDWA contaminants

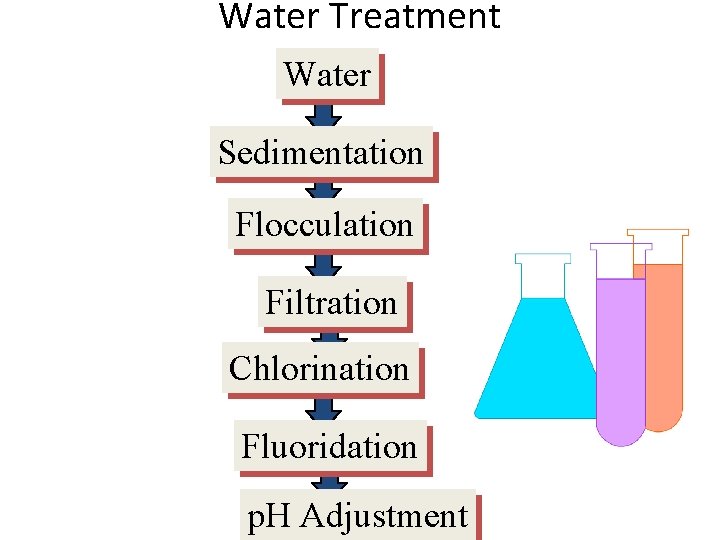
Water Treatment Water Sedimentation Flocculation Filtration Chlorination Fluoridation p. H Adjustment

Sedimentation The water is pumped into the bottom of the sedimentation tanks, so as not to disturb the clearer water at the top. The suspended particles settle to the bottom.

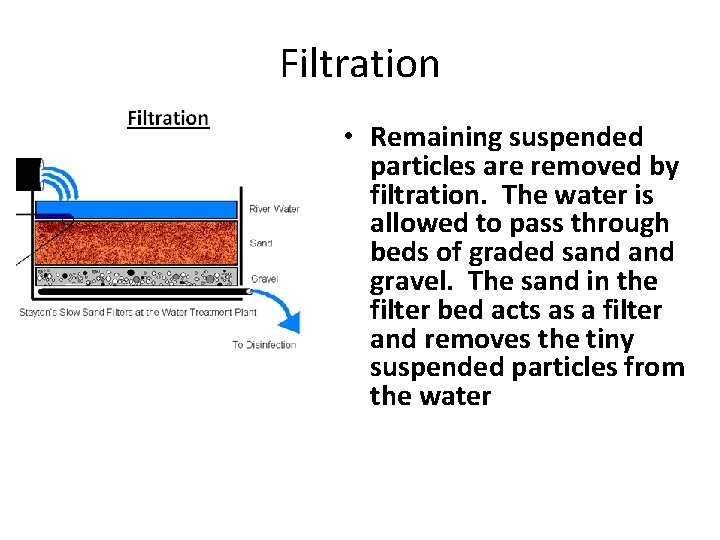
Filtration • Remaining suspended particles are removed by filtration. The water is allowed to pass through beds of graded sand gravel. The sand in the filter bed acts as a filter and removes the tiny suspended particles from the water

Chlorination This is the addition of chlorine or chlorine compounds to kill microorganisms in the water and to prevent reinfection. Both chlorine and sodium hypochlorite are added to the water to form an active disinfecting agent in the water

Fluoridation This is the addition of fluoride compounds to drinking water to prevent tooth decay. Sodium fluorosilicate is usually the fluoridating agent.

p. H adjustment It may be necessary to adjust the p. H of the water before it leaves the treatment plant. Tap water should have a p. H in the range of 6 – 8. If the water is too acidic, lime is added to raise the p. H and if the p. H is too high, sulfuric acid is added.

Sewage Treatment • Primary Treatment • Physical Process • Secondary Treatment • Biological Process • Tertiary Treatment • Chemical Process

Primary Treatment Solids and large floating debris are screened from the waste water Remaining solids are removed by allowing the waste to settle in sedimentation tanks

Secondary Treatment • Activated Sludge Method • The activated sludge method operates aerobically. The sewage is fed continuously into aerated tanks that is kept oxygenated by mechanical agitators. Aerobic Microorganisms break down organic waste in the sewage by oxidation in air to carbon dioxide and water

Tertiary Treatment Removal of nitrates and phosphates Phosphates are removed by precipitation with Aluminium Phosphate Nitrates are removed by biological nitrification. Tertiary treatment is a costly process

Eutrophication of Water • Eutrophication is caused by the overenrichment of water by nutrients such as phosphates and nitrates.

Eutrophication of Water • In effect, the nutrients behave as fertilisers that increase the growth of plants such as algae in lakes and rivers • The algae are short lived. As they decay, much of the dissolved oxygen in the water is used up, leading to the death of many forms of animal life.

Nitrate Fertilisers • Waterways can also be polluted by the run-off of excess fertiliser from farmland. Excess nitrate in the fertiliser may be washed into rivers or lakes by rainwater. Eutrophication will result.

Pollution of Heavy Metals • Metals with high relative atomic masses such as mercury, cadmium and lead are known as heavy metals. • When recycling is inadequate, quantities of these elements are dumped e. g. Car batteries containing lead or dry batteries containing cadmium.

Pollution of Heavy Metals • Dipositive ions of these metals • e. g. Hg 2+ , Cd 2+ , and Pb 2+ sometimes get into waterways from industrial effluent and consequently into drinking water. • These elements are cumulative poisons in that frequent exposure causes build up in the body, resulting in serious health damage. • Lead ions can be removed from the effluent by precipitation

EU Legislation on Water Quality • There are limits to the quantities of Hg 2+ , Cd 2+ , and Pb 2+ ions that can be tolerated in waterways because of their toxic effects. • Limits on phosphates and nitrates help to reduce the occurrence of eutrophication in waterways. • Limits are also set for chemical species dissolve in drinking water.
 300+200+200
300+200+200 Factors of 500
Factors of 500 100 200 300
100 200 300 200 300 300
200 300 300 Scientific notation
Scientific notation 300+300+400
300+300+400 300+300+400
300+300+400 300 + 300 + 400
300 + 300 + 400 300+300+400
300+300+400 400 + 300 + 300
400 + 300 + 300 300 300 400
300 300 400 300+300+400
300+300+400 200+200+300+300
200+200+300+300 Water and water and water water
Water and water and water water Differentiate between average speed and average velocity
Differentiate between average speed and average velocity Alex pulls on the handle of a claw hammer
Alex pulls on the handle of a claw hammer A crane uses an average force of 5200 n
A crane uses an average force of 5200 n A crane uses an average force of 5200 n
A crane uses an average force of 5200 n Materials that are harmful and useful
Materials that are harmful and useful Microbes in household products
Microbes in household products Household composition letter
Household composition letter Nursing metric conversions
Nursing metric conversions What's household composition
What's household composition Household product symbols
Household product symbols Household and firm
Household and firm For protection household circuits contain
For protection household circuits contain