Water Treatment Monroe L WeberShirk School of Civil

Water Treatment Monroe L. Weber-Shirk School of Civil and Environmental Engineering

Reflections Ø What are the two broad tasks of environmental engineers? Ø What is the connection between the broad tasks of environmental engineers and building a water treatment plant? Ø Why may the water need to be changed/treated?

Simple Sorting Ø Goal: clean water Ø Source: (contaminated) surface water Ø Solution: separate contaminants from water Ø How?

Where are we going? Ø Unit processes* designed to particles Ø remove ___________ dissolved chemicals Ø inactivate _____ pathogens Ø *Unit process: a process that is used in similar ways in many different applications Ø sedimentation Ø filtration Ø. . .

Unit Processes Designed to Remove Particulate Matter Ø Screening Ø Sedimentation Ø Coagulation/flocculation Ø Filtration Øslow sand filters Ørapid sand filters Ødiatomaceous earth filters Ømembrane filters
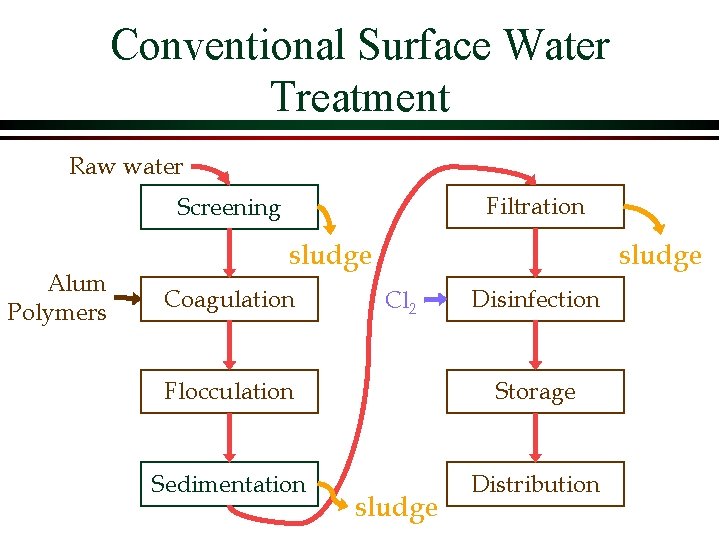
Conventional Surface Water Treatment Raw water Filtration Screening Alum Polymers sludge Coagulation sludge Cl 2 Disinfection Flocculation Storage Sedimentation Distribution sludge
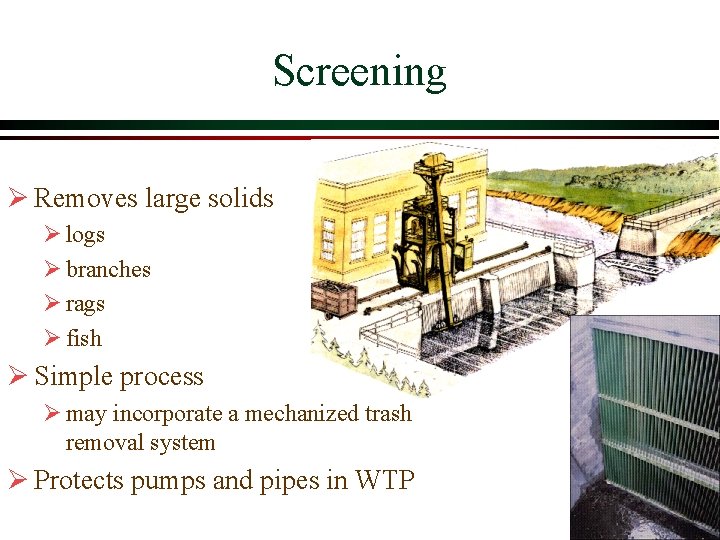
Screening Ø Removes large solids Ø logs Ø branches Ø rags Ø fish Ø Simple process Ø may incorporate a mechanized trash removal system Ø Protects pumps and pipes in WTP

Sedimentation Ø the oldest form of water treatment Ø uses gravity to separate particles from water Ø often follows coagulation and flocculation Ø occurs in NYC’s _____ reservoirs
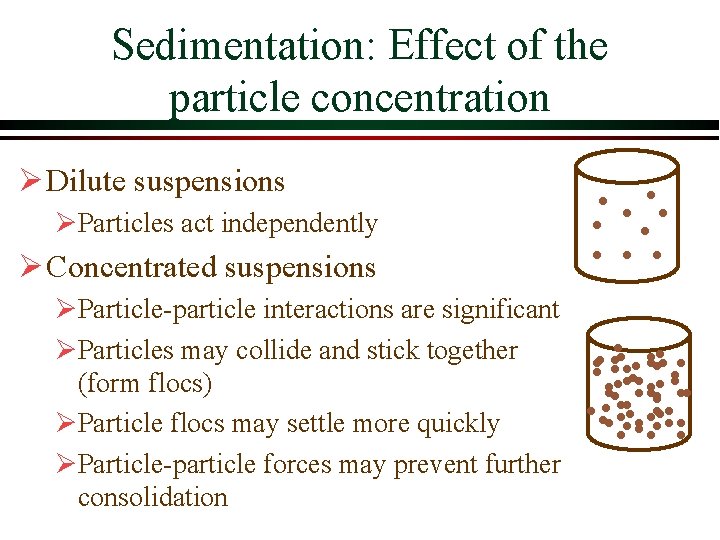
Sedimentation: Effect of the particle concentration Ø Dilute suspensions ØParticles act independently Ø Concentrated suspensions ØParticle-particle interactions are significant ØParticles may collide and stick together (form flocs) ØParticle flocs may settle more quickly ØParticle-particle forces may prevent further consolidation

How fast do particles fall in dilute suspensions? Ø What are the important parameters? ØInitial conditions ØAfter falling for some time. . . Ø What are the important forces? Ø_____ Gravity Fluid drag Ø_____
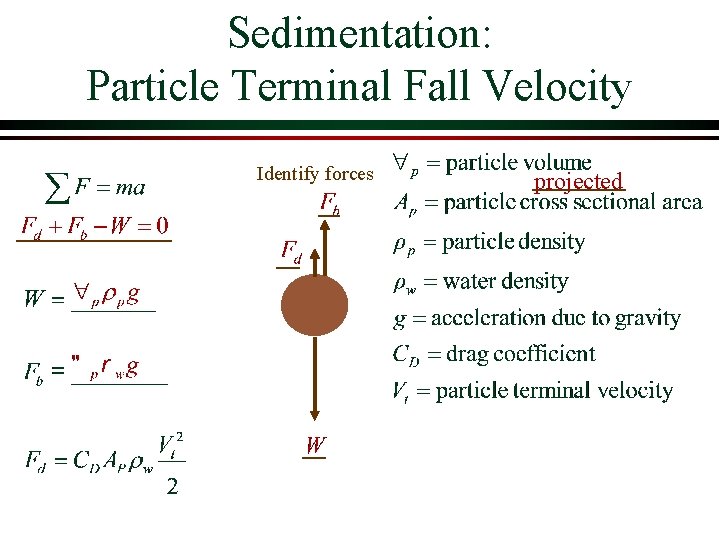
Sedimentation: Particle Terminal Fall Velocity Identify forces projected
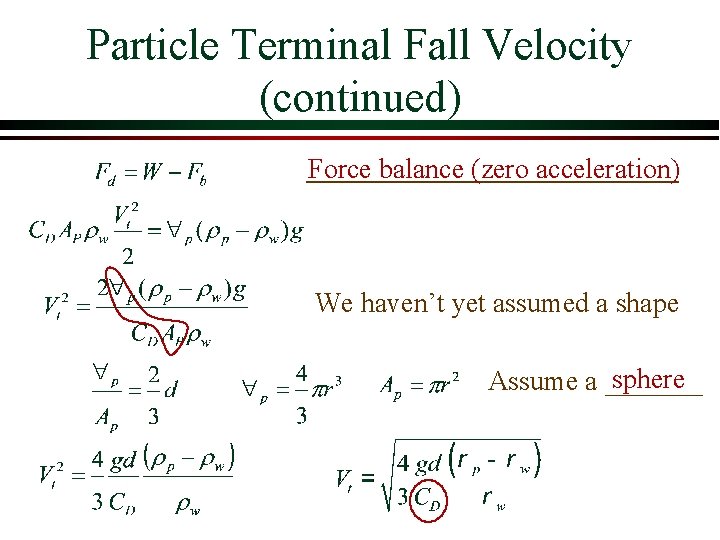
Particle Terminal Fall Velocity (continued) Force balance (zero acceleration) We haven’t yet assumed a shape sphere Assume a _______
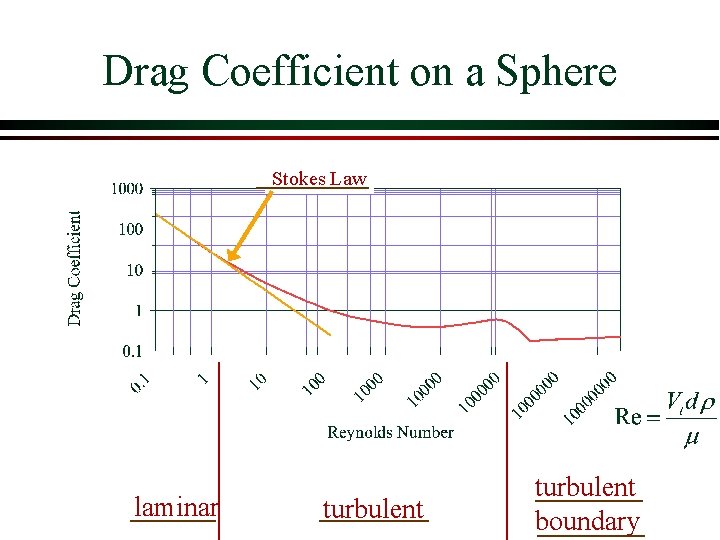
Drag Coefficient on a Sphere Stokes Law laminar turbulent boundary
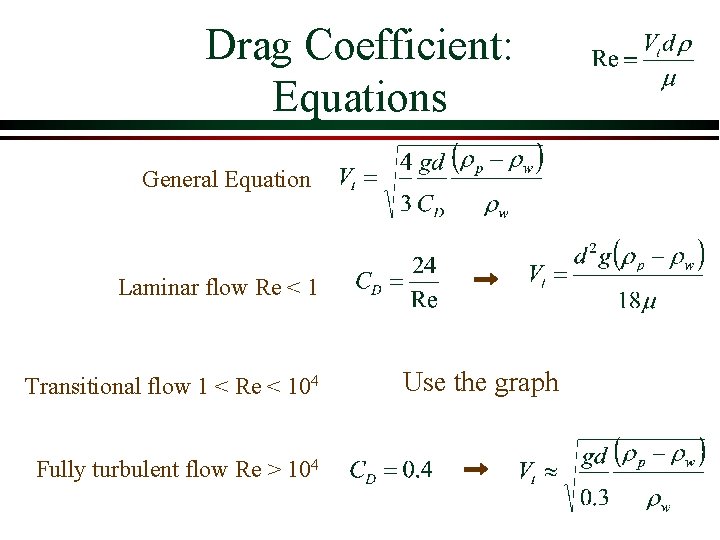
Drag Coefficient: Equations General Equation Laminar flow Re < 1 Transitional flow 1 < Re < 104 Fully turbulent flow Re > 104 Use the graph
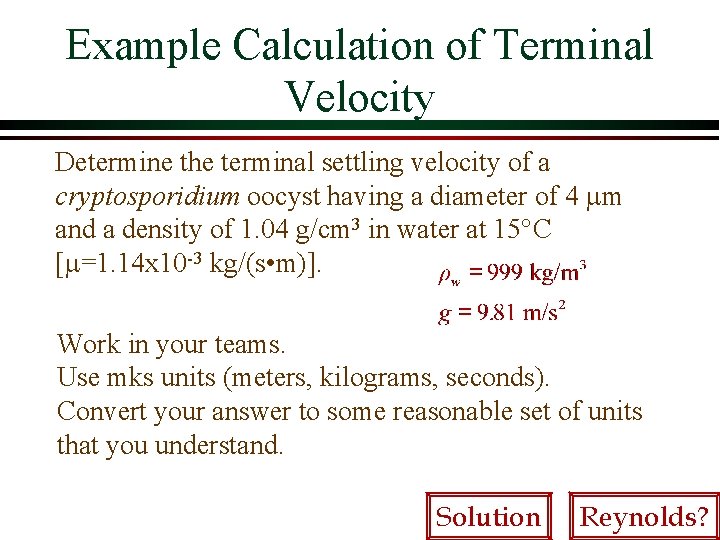
Example Calculation of Terminal Velocity Determine the terminal settling velocity of a cryptosporidium oocyst having a diameter of 4 mm and a density of 1. 04 g/cm 3 in water at 15°C [m=1. 14 x 10 -3 kg/(s • m)]. Work in your teams. Use mks units (meters, kilograms, seconds). Convert your answer to some reasonable set of units that you understand. Solution Reynolds?
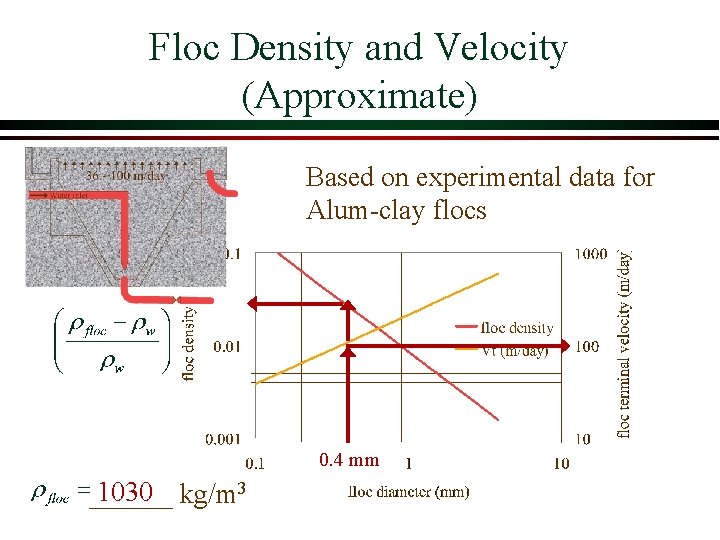
Floc Density and Velocity (Approximate) Based on experimental data for Alum-clay flocs 0. 4 mm 1030 kg/m 3 ______
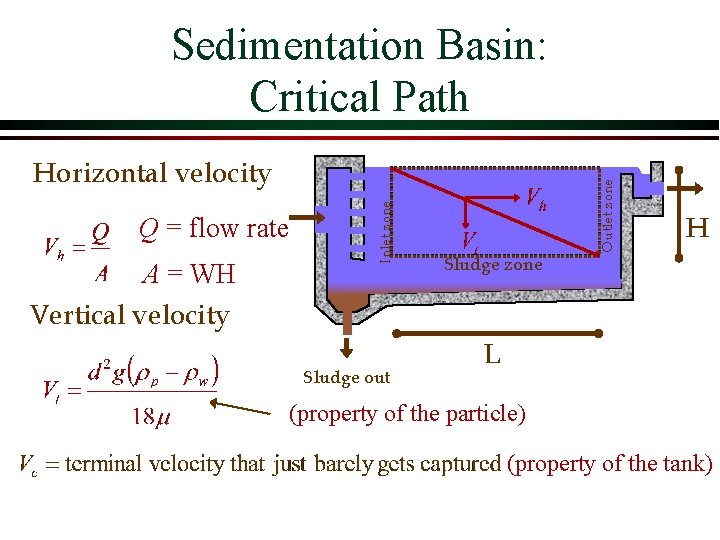
Sedimentation Basin: Critical Path A = WH Vertical velocity Sludge out Outlet zone Q = flow rate Inlet zone Horizontal velocity H Sludge zone L (property of the particle) (property of the tank)
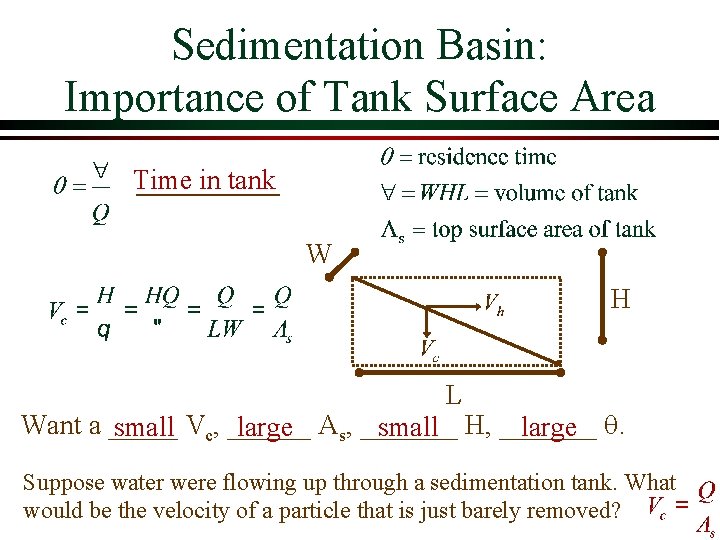
Sedimentation Basin: Importance of Tank Surface Area Time in tank W H L Want a _____ small Vc, ______ large As, _______ small H, _______ large q. Suppose water were flowing up through a sedimentation tank. What would be the velocity of a particle that is just barely removed?
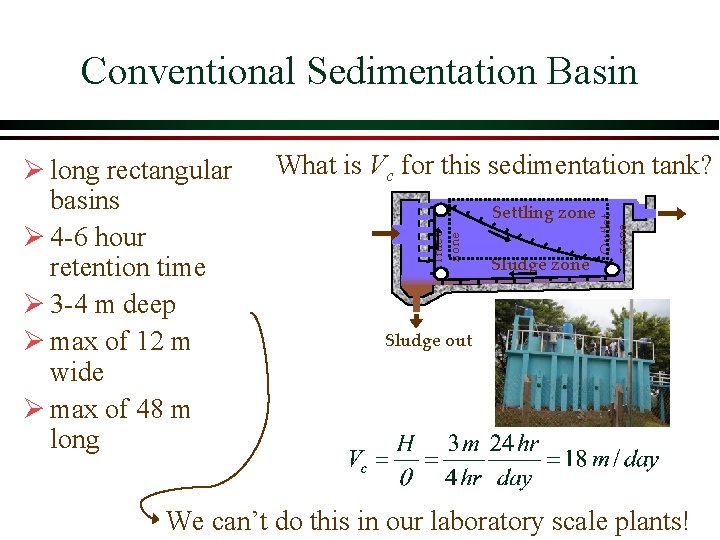
Conventional Sedimentation Basin Settling zone Sludge zone Outlet zone What is Vc for this sedimentation tank? Inlet zone Ø long rectangular basins Ø 4 -6 hour retention time Ø 3 -4 m deep Ø max of 12 m wide Ø max of 48 m long Sludge out We can’t do this in our laboratory scale plants!

Outlet zone Inlet zone Design Criteria for Sedimentation Tanks Settling zone Sludge zone Ø ________________ Minimal turbulence (inlet baffles) Ø ________________ Uniform velocity (small dimensions normal to velocity) Ø ________________ No scour of settled particles Ø ________________ Slow moving particle collection system Ø ________________ Q/As must be small (to capture small particles) This will be one of the ways you can improve the performance of your water treatment plant.
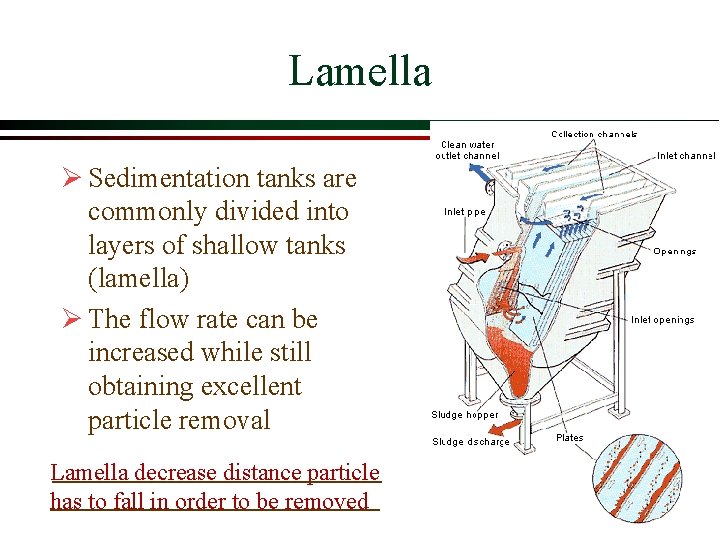
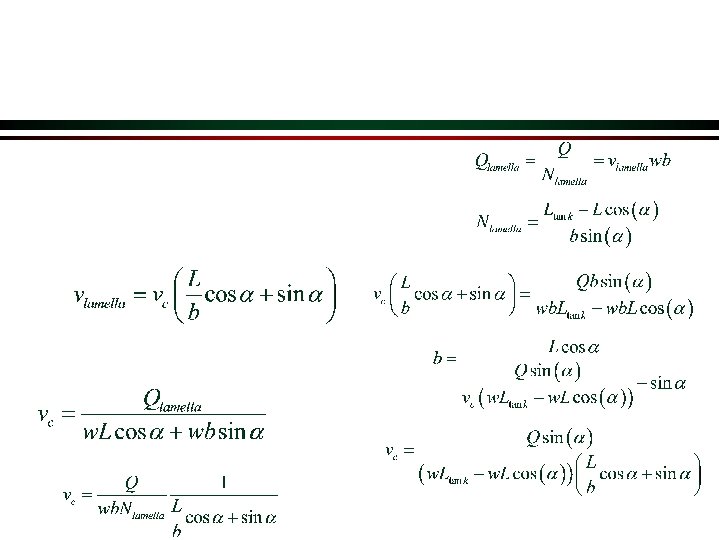
Lamella Ø Sedimentation tanks are commonly divided into layers of shallow tanks (lamella) Ø The flow rate can be increased while still obtaining excellent particle removal Lamella decrease distance particle has to fall in order to be removed
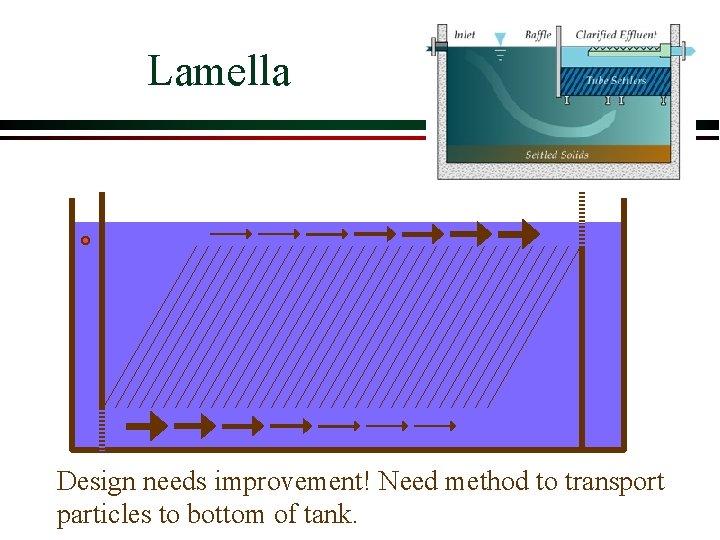
Lamella Design needs improvement! Need method to transport particles to bottom of tank.
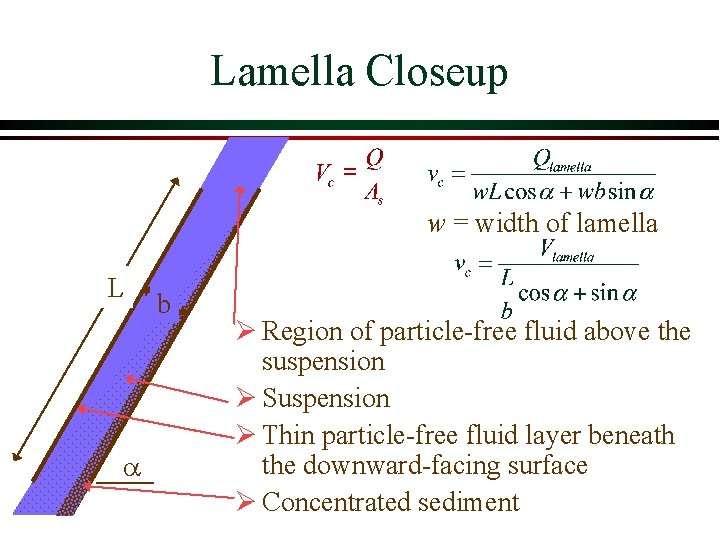
Lamella Closeup w = width of lamella L a b Ø Region of particle-free fluid above the suspension Ø Suspension Ø Thin particle-free fluid layer beneath the downward-facing surface Ø Concentrated sediment
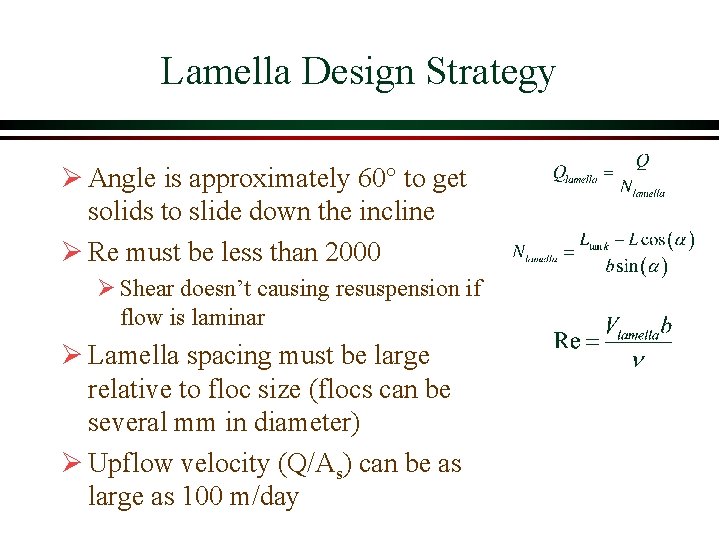
Lamella Design Strategy Ø Angle is approximately 60° to get solids to slide down the incline Ø Re must be less than 2000 Ø Shear doesn’t causing resuspension if flow is laminar Ø Lamella spacing must be large relative to floc size (flocs can be several mm in diameter) Ø Upflow velocity (Q/As) can be as large as 100 m/day
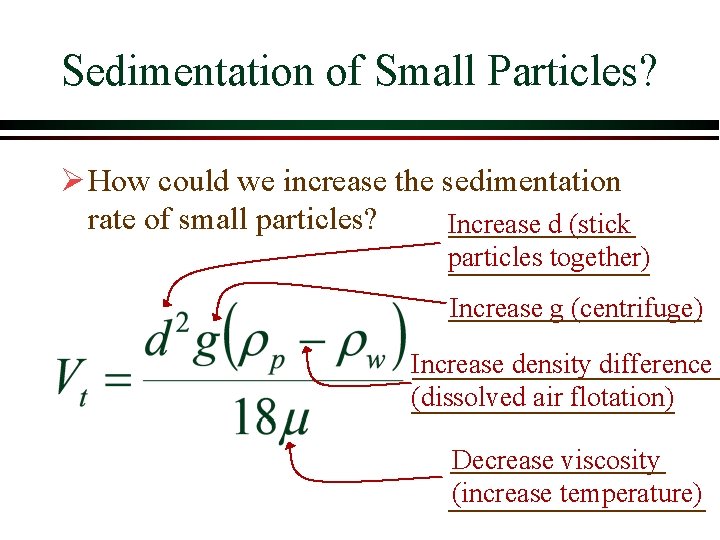
Sedimentation of Small Particles? Ø How could we increase the sedimentation rate of small particles? Increase d (stick particles together) Increase g (centrifuge) Increase density difference (dissolved air flotation) Decrease viscosity (increase temperature)
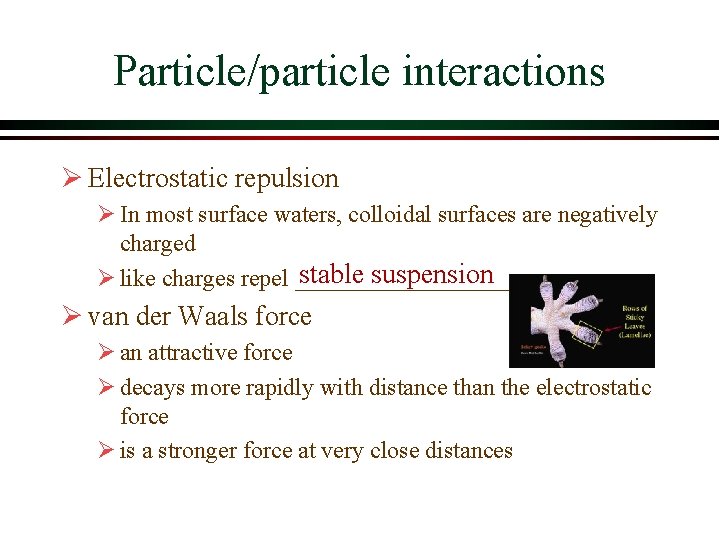
Particle/particle interactions Ø Electrostatic repulsion Ø In most surface waters, colloidal surfaces are negatively charged stable suspension Ø like charges repel _________ Ø van der Waals force Ø an attractive force Ø decays more rapidly with distance than the electrostatic force Ø is a stronger force at very close distances
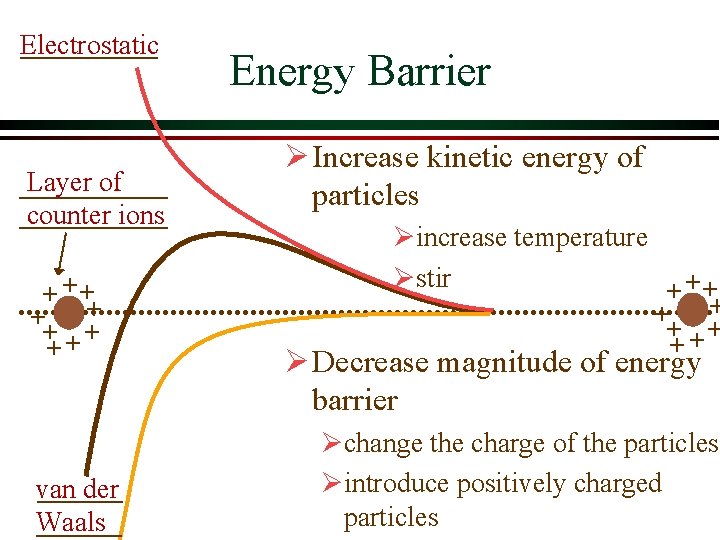
Electrostatic Layer of counter ions + ++ + van der Waals Energy Barrier Ø Increase kinetic energy of particles Øincrease temperature Østir + ++ + Ø Decrease magnitude of energy barrier Øchange the charge of the particles Øintroduce positively charged particles
Coagulation Ø Coagulation is a physical-chemical process whereby particles are destabilized Ø Several mechanisms Øadsorption of cations onto negatively charged particles Ødecrease thickness of the layer of counter ions Øsweep coagulation Øinterparticle bridging
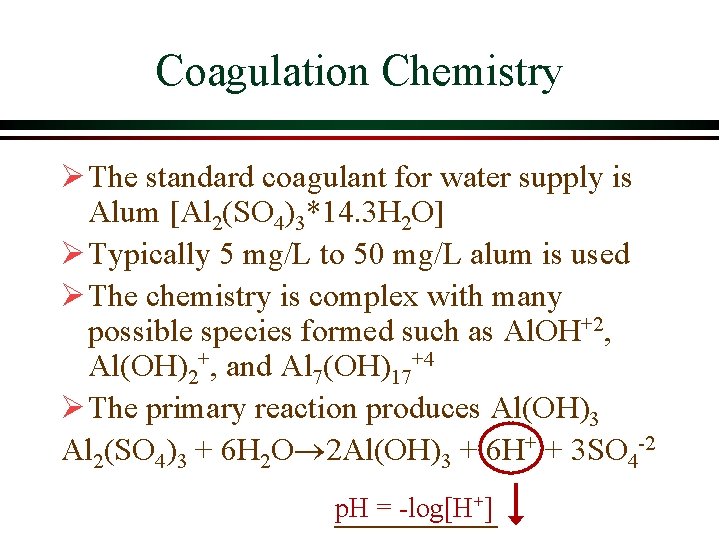
Coagulation Chemistry Ø The standard coagulant for water supply is Alum [Al 2(SO 4)3*14. 3 H 2 O] Ø Typically 5 mg/L to 50 mg/L alum is used Ø The chemistry is complex with many possible species formed such as Al. OH+2, Al(OH)2+, and Al 7(OH)17+4 Ø The primary reaction produces Al(OH)3 Al 2(SO 4)3 + 6 H 2 O 2 Al(OH)3 + 6 H+ + 3 SO 4 -2 p. H = -log[H+]
![Coagulation Chemistry Ø Aluminum hydroxide [Al(OH)3] forms amorphous, gelatinous flocs that are heavier than Coagulation Chemistry Ø Aluminum hydroxide [Al(OH)3] forms amorphous, gelatinous flocs that are heavier than](http://slidetodoc.com/presentation_image_h/0deabb099b625b54e807e85775da2847/image-31.jpg)
Coagulation Chemistry Ø Aluminum hydroxide [Al(OH)3] forms amorphous, gelatinous flocs that are heavier than water Ø The flocs look like snow in water Ø These flocs entrap particles as the flocs settle (sweep coagulation)

Coagulant introduction with rapid mixing Ø The coagulant must be mixed with the water Ø Retention times in the mixing zone are typically between 1 and 10 seconds Ø Types of rapid mix units Ø pumps Ø hydraulic jumps Ø flow-through basins with many baffles Ø In-line blenders Ø In-line static mixers
Flocculation Ø Coagulation has destabilized the particles by reducing the energy barrier Ø Now we want to get the particles to collide Ø We need relative motion between particles Ø________ (effective for particles Brownian motion smaller than 1 mm) Ø_____ Differential _______ sedimentation (big particles hit smaller particles) Ø_______ Shear
Mechanical Flocculation Ø Shear provided by turbulence created by gentle stirring Ø Turbulence also keeps large flocs from settling so they can grow even larger! Ø Retention time of 10 - 30 minutes Ø Advantage is that amount of shear can be varied independent of flow rate Ø Disadvantage is the tanks are far from plug flow
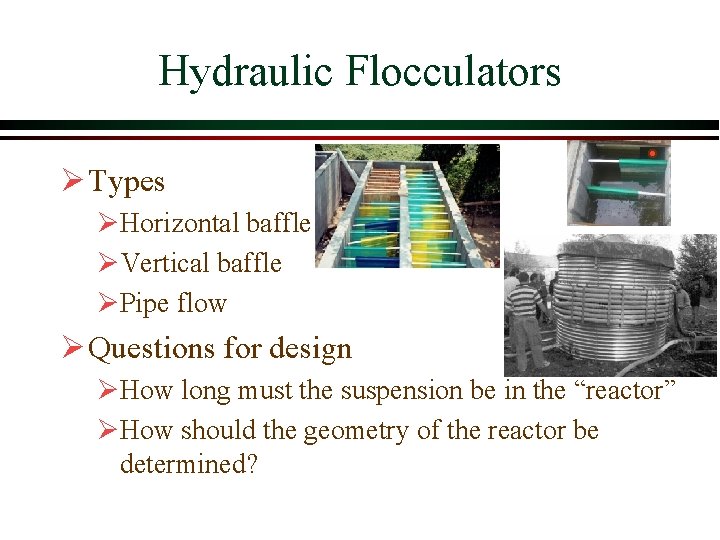
Hydraulic Flocculators Ø Types ØHorizontal baffle ØVertical baffle ØPipe flow Ø Questions for design ØHow long must the suspension be in the “reactor” ØHow should the geometry of the reactor be determined?
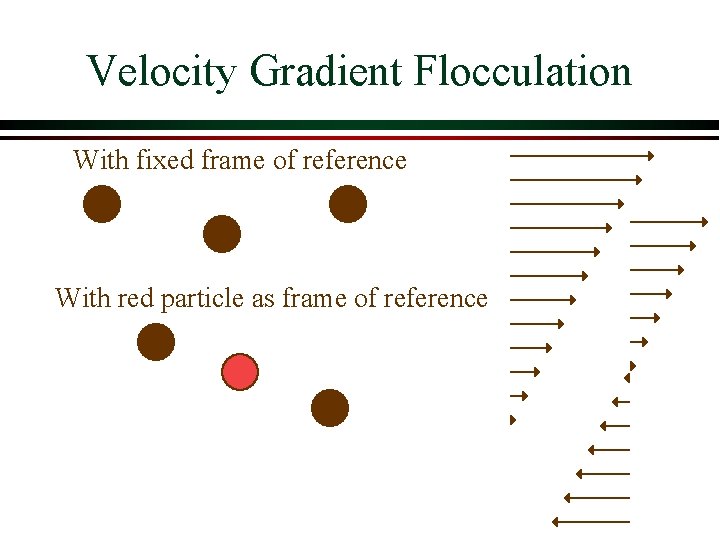
Velocity Gradient Flocculation With fixed frame of reference With red particle as frame of reference
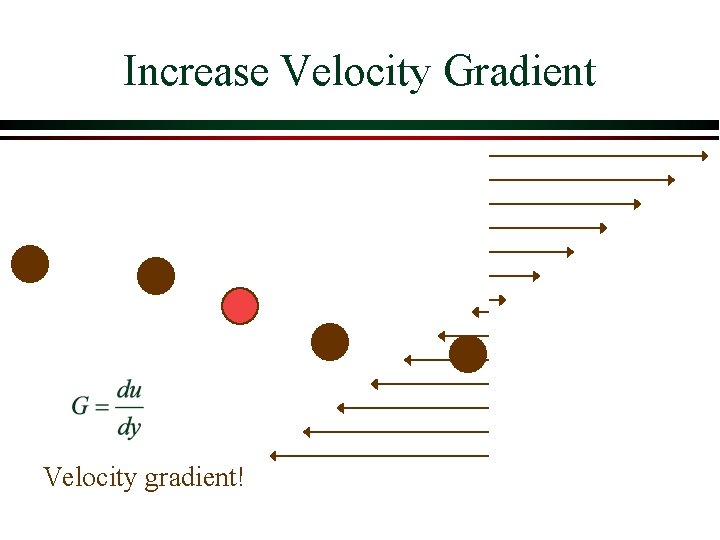
Increase Velocity Gradient Velocity gradient!
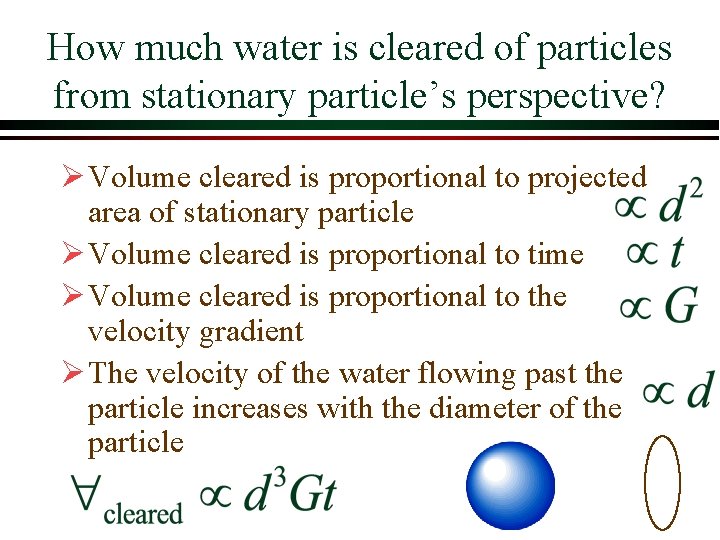
How much water is cleared of particles from stationary particle’s perspective? Ø Volume cleared is proportional to projected area of stationary particle Ø Volume cleared is proportional to time Ø Volume cleared is proportional to the velocity gradient Ø The velocity of the water flowing past the particle increases with the diameter of the particle

How much volume must be cleared before a collision occurs? Ø What is the average volume of water occupied by a particle? Ø Given C mg/L of particles in suspension… Ø Need to know particle diameter (d) Ø And density (rparticles) Ø How many particles are in a volume of water?
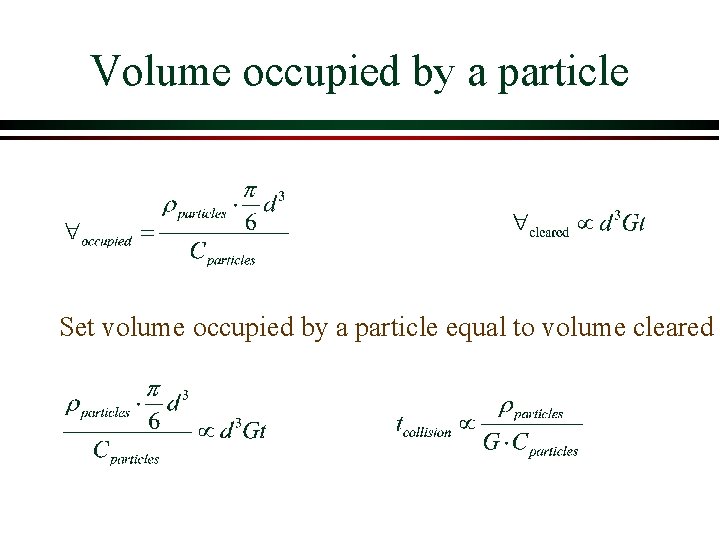
Volume occupied by a particle Set volume occupied by a particle equal to volume cleared

Collision Time Ø A measure of how long the particles must be in the velocity gradient to double in size Ø A series of collisions must occur for particles to grow large enough to be easily removed by sedimentation
Flocculation Reactor Design Ø Critical design is when particle concentration is low Ø Higher velocity gradients would decrease the characteristic collision time Ø Why not design a tiny reactor with huge velocity gradients? Ø SHEAR
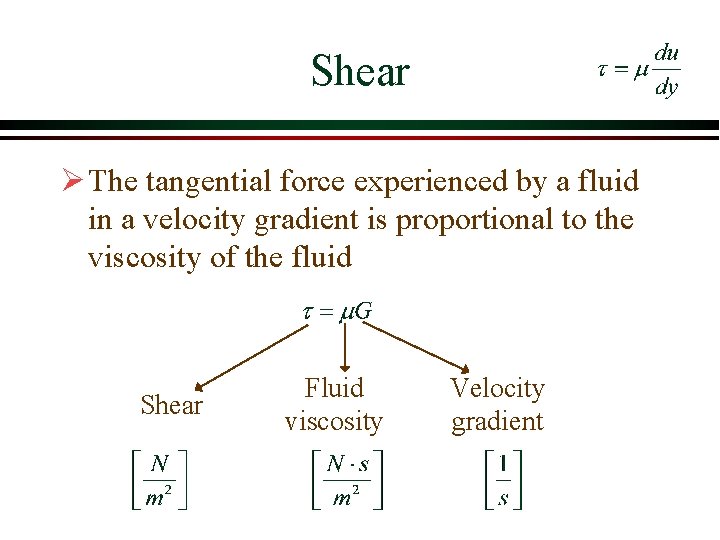
Shear Ø The tangential force experienced by a fluid in a velocity gradient is proportional to the viscosity of the fluid Shear Fluid viscosity Velocity gradient

Too much shear? Ø Flocs can be broken by too much shear Ø Amazingly, we haven’t been able to find good information on the shear level that causes aluminum-clay flocs to breakup Ø fine grained cohesive sediments within estuarine waters were shown to produce smaller flocs when the shear exceeded 0. 35 Pa (equivalent to a G of approximately 400/s)
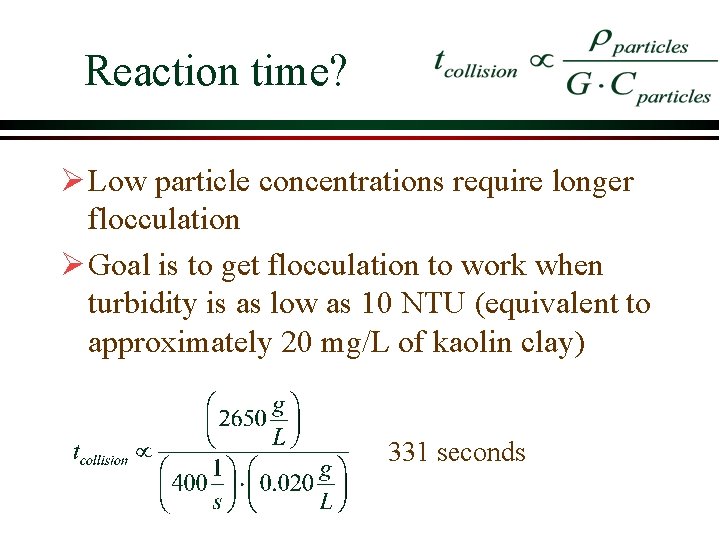
Reaction time? Ø Low particle concentrations require longer flocculation Ø Goal is to get flocculation to work when turbidity is as low as 10 NTU (equivalent to approximately 20 mg/L of kaolin clay) 331 seconds
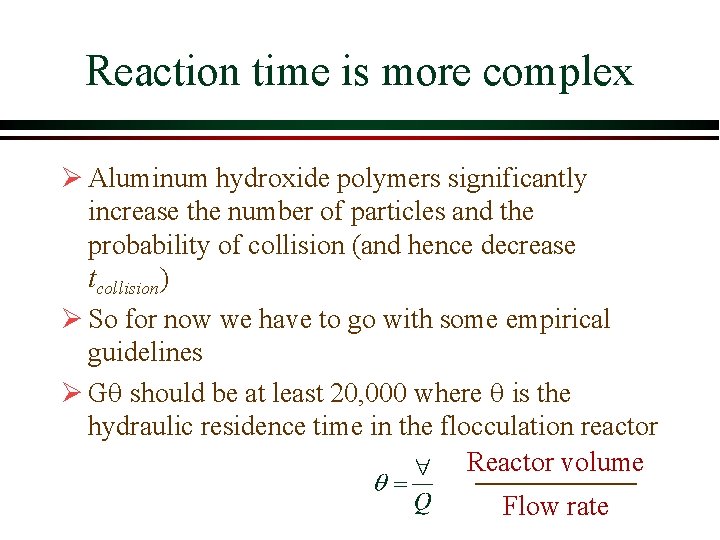
Reaction time is more complex Ø Aluminum hydroxide polymers significantly increase the number of particles and the probability of collision (and hence decrease tcollision) Ø So for now we have to go with some empirical guidelines Ø Gq should be at least 20, 000 where q is the hydraulic residence time in the flocculation reactor Reactor volume Flow rate
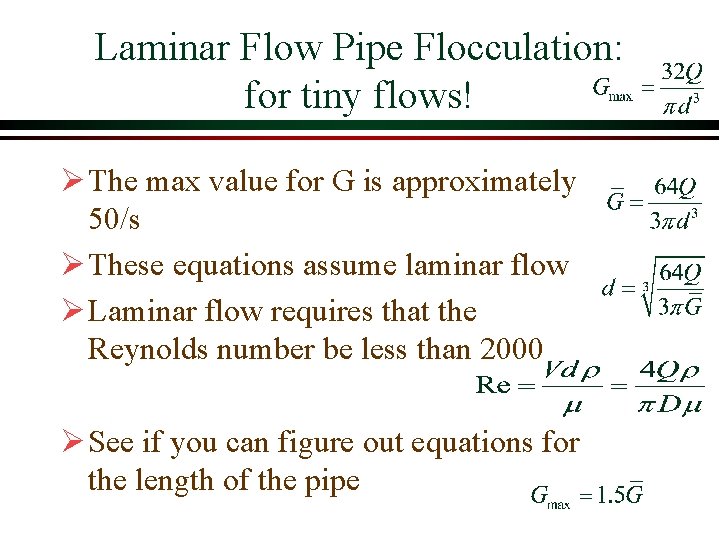
Laminar Flow Pipe Flocculation: for tiny flows! Ø The max value for G is approximately 50/s Ø These equations assume laminar flow Ø Laminar flow requires that the Reynolds number be less than 2000 Ø See if you can figure out equations for the length of the pipe
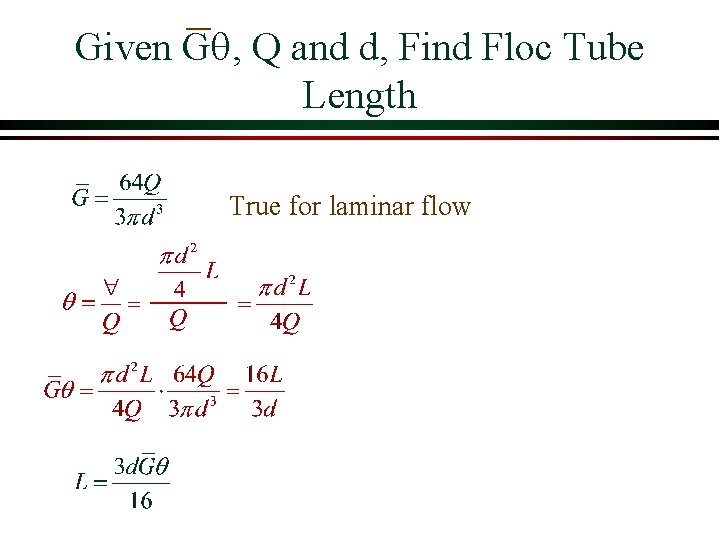
Given Gq, Q and d, Find Floc Tube Length True for laminar flow
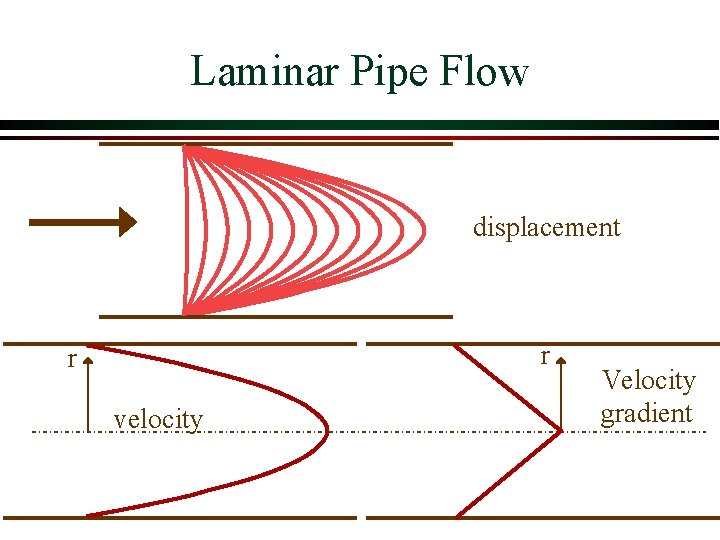
Laminar Pipe Flow displacement r r velocity Velocity gradient
Coagulation/Flocculation Ø Inject Coagulant in rapid mixer Ø Water flows from rapid mix unit into flocculation reactor Ø Water flows from flocculation reactor into sedimentation tank Ømake sure flocs don’t break! Øflocs settle and are removed

Jar Test Ø Mimics the rapid mix, flocculation, sedimentation treatment steps in a beaker Ø Allows operator to test the effect of different coagulant dosages or of different coagulants Ø Low tech water bottle test

Unit Processes in Conventional Surface Water Treatment Ø We’ve covered ØSedimentation ØCoagulation/flocculation Ø Coming up! ØFiltration ØDisinfection ØRemoval of Dissolved Substances
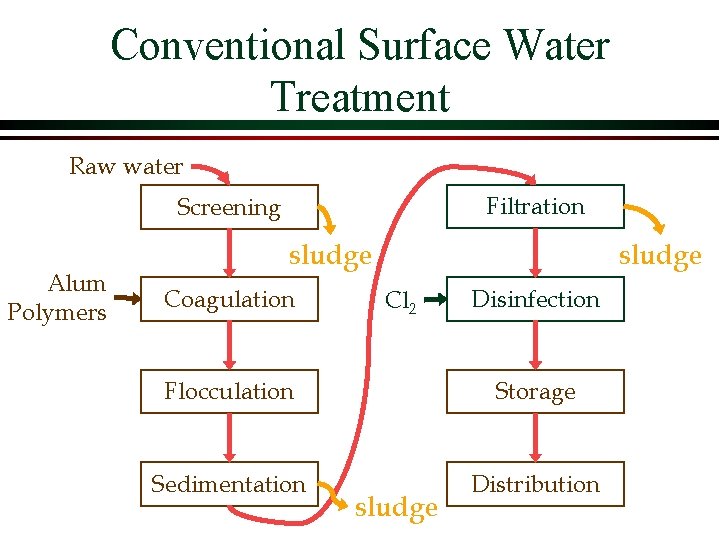
Conventional Surface Water Treatment Raw water Filtration Screening Alum Polymers sludge Coagulation sludge Cl 2 Disinfection Flocculation Storage Sedimentation Distribution sludge

Filtration Ø Slow sand filters Ø Diatomaceous earth filters Ø Membrane filters Ø Rapid sand filters (Conventional Treatment)

Slow Sand Filtration Ø First filters to be used on a widespread basis Ø Fine sand with an effective size of 0. 2 mm Ø Low flow rates (10 - 40 cm/hr) Ø Schmutzdecke (_____) forms on top of filter cake the filter Øcauses high head loss Ømust be removed periodically Ø Used without coagulation/flocculation!

Diatomaceous Earth Filters Ø Diatomaceous earth (DE) is made of the silica skeletons of diatoms Ø DE is added to water and then fed to a special microscreen Ø The DE already on the microscreen strains particles and DE from the water Ø The continuous DE feed prevents the gradually thickening DE cake from developing excessive head loss Ø Was seriously considered for Croton Filtration Plant
Membrane Filters Ø Much like the membrane filters used to enumerate coliforms Ømuch greater surface area Ø Produce very high quality water (excellent particle removal) Ø Clog rapidly if the influent water is not of sufficiently high quality Ø More expensive than sand DE filters
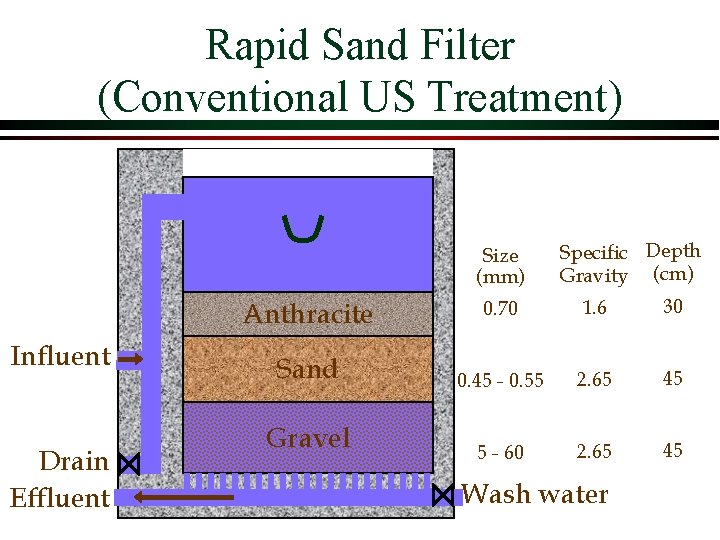
Rapid Sand Filter (Conventional US Treatment) Size (mm) Anthracite Influent Drain Effluent Sand Gravel Specific Depth Gravity (cm) 0. 70 1. 6 30 0. 45 - 0. 55 2. 65 45 5 - 60 2. 65 45 Wash water
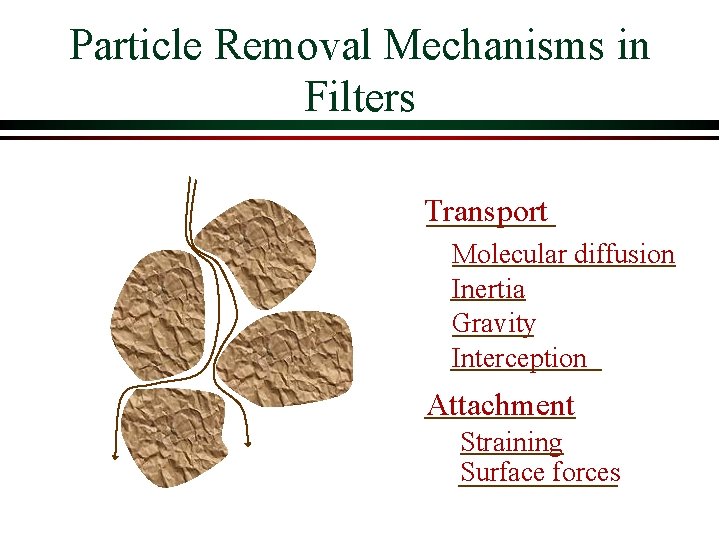
Particle Removal Mechanisms in Filters Transport Molecular diffusion Inertia Gravity Interception Attachment Straining Surface forces
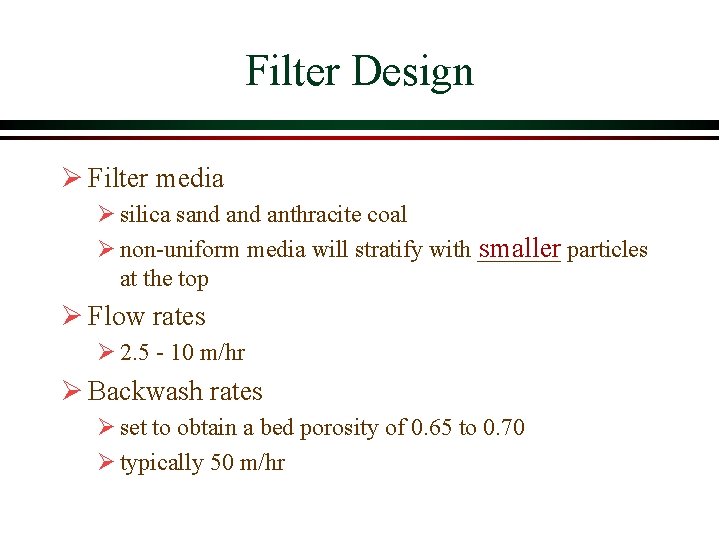
Filter Design Ø Filter media Ø silica sand anthracite coal smaller particles Ø non-uniform media will stratify with _______ at the top Ø Flow rates Ø 2. 5 - 10 m/hr Ø Backwash rates Ø set to obtain a bed porosity of 0. 65 to 0. 70 Ø typically 50 m/hr
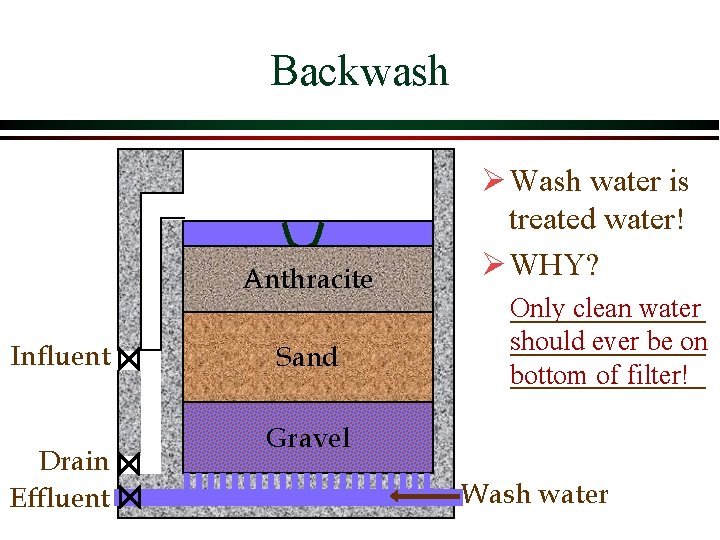
Backwash Anthracite Influent Drain Effluent Sand Ø Wash water is treated water! Ø WHY? Only clean water should ever be on bottom of filter! Gravel Wash water

Ways to Improve Filtration Ø Filter to waste Ø Extended Terminal Sub-fluidization Wash Ø Alum feed directly to filter? Ø Potato starch?

Disinfection Ø Disinfection: operations aimed at killing or inactivating pathogenic microorganisms ______ Ø Ideal disinfectant Toxic to pathogens Ø________ Not toxic to humans Ø________ Fast rate of kill Ø________ Residual protection Ø________ Economical Ø________

Disinfection Options Ø Chlorine Ø chlorine gas Poisonous gas – risk of a leak Ø sodium hypochlorite (bleach) Ø Ozone Ø Irradiation with Ultraviolet light Ø Sonification Ø Electric Current Ø Gamma-ray irradiation
Chlorine Ø First large-scale chlorination was in 1908 at the Boonton Reservoir of the Jersey City Water Works in the United States Chlorine oxidizes Ø Widely used in the US organic matter Ø Typical dosage (1 -5 mg/L) Ø variable, based on the chlorine demand Ø goal of 0. 2 mg/L residual Ø Trihalomethanes (EPA primary standard is 0. 08 Pathogen/carcinogen tradeoff mg/L)
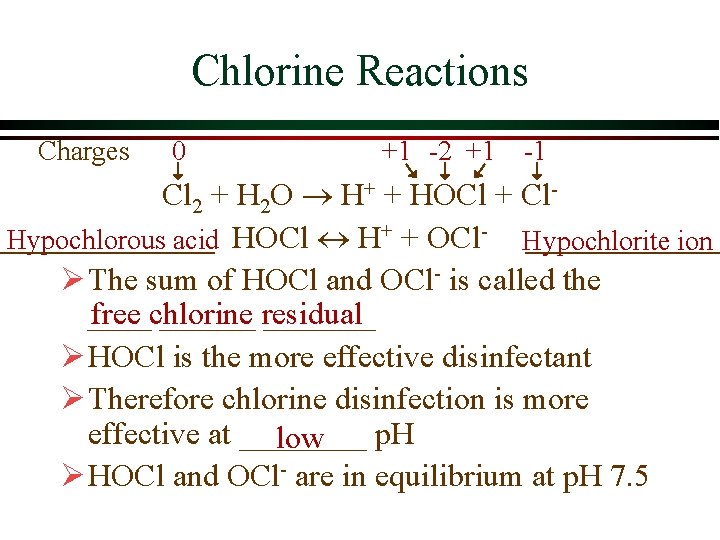
Chlorine Reactions Charges 0 +1 -2 +1 -1 Cl 2 + H 2 O H+ + HOCl + Cl. Hypochlorous acid HOCl H+ + OCl- Hypochlorite ion Ø The sum of HOCl and OCl- is called the free chlorine ______ residual _______ Ø HOCl is the more effective disinfectant Ø Therefore chlorine disinfection is more effective at ____ p. H low Ø HOCl and OCl- are in equilibrium at p. H 7. 5

EPA Pathogen Inactivation Requirements Safe Drinking Water Act Ø SDWA requires 99. 9% inactivation for Giardia and 99. 99% inactivation of viruses Ø Giardia is more difficult to kill with chlorine than viruses and thus Giardia inactivation determines the CT Concentration x Time Enumerating Giardia is difficult, time-consuming and costly. How would you ensure that water treatment plants meet this criteria? Where are Giardia removed/inactivated?
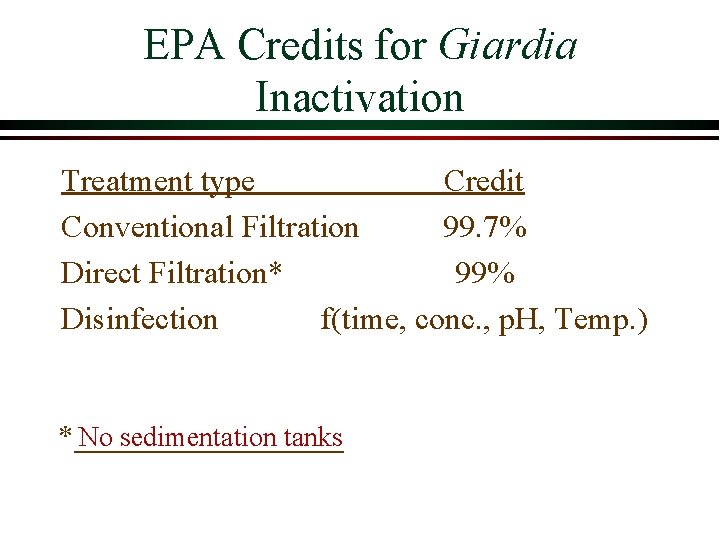
EPA Credits for Giardia Inactivation Treatment type Credit Conventional Filtration 99. 7% Direct Filtration* 99% Disinfection f(time, conc. , p. H, Temp. ) * No sedimentation tanks
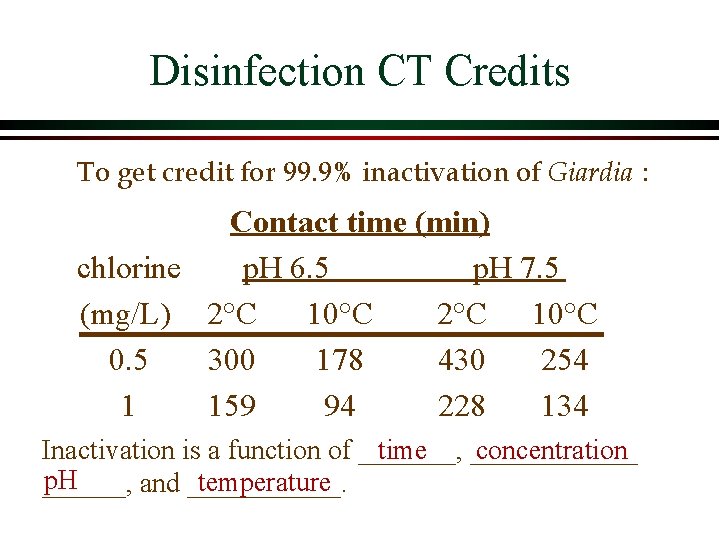
Disinfection CT Credits To get credit for 99. 9% inactivation of Giardia : Contact time (min) chlorine p. H 6. 5 p. H 7. 5 (mg/L) 2°C 10°C 0. 5 300 178 430 254 1 159 94 228 134 Inactivation is a function of _______, time ______ concentration p. H temperature ______, and ______.
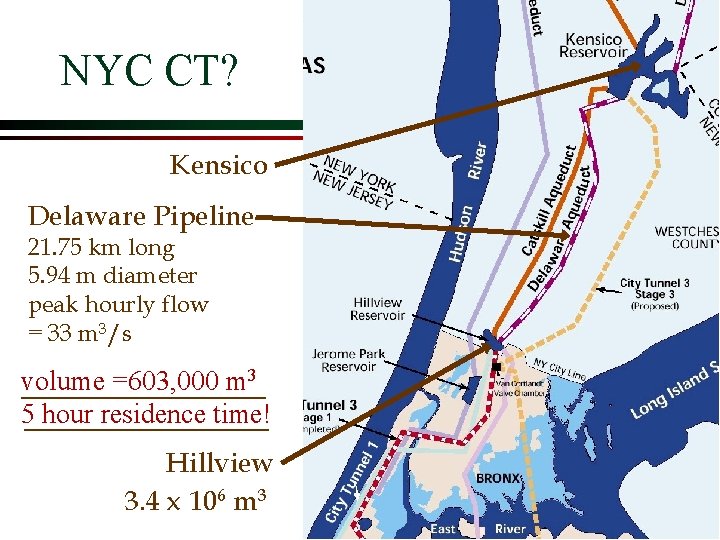
NYC CT? Kensico Delaware Pipeline 21. 75 km long 5. 94 m diameter peak hourly flow = 33 m 3/s volume =603, 000 m 3 5 hour residence time! Hillview 3. 4 x 106 m 3

NYC CT Problem Ø Hillview Reservoir is an open reservoir Ø Should the chlorine contact time prior to arrival at Hillview count? Giardia contamination from Upstate Reservoirs will be decreased, but recontamination at Hillview is possible

Ozone Ø Widely used in Europe Ø O 3 is chemically unstable Ø Must be produced on site Ø More expensive than chlorine (2 - 3 times) Ø Typical dosages range from 1 to 5 mg/L Ø Often followed by chlorination so that the residual chlorine can provide a protective _______

Removal of Dissolved Substances (1) Ø Aeration (before filtration) Øoxidizes iron or manganese in groundwater Øoxidized forms are less soluble and thus precipitate out of solution Øremoves hydrogen sulfide (H 2 S) Ø Softening (before filtration) Øused to remove Ca+2 and Mg+2 Øusually not necessary with surface waters

Removal of Dissolved Substances (2) Ø Activated Carbon (between filtration and disinfection) Ø extremely adsorbent Ø used to remove organic contaminants Ø spent activated carbon can be regenerated with superheated steam Ø Reverse Osmosis Ø semi-permeable membrane allows water molecules to pass, but not the larger ions and molecules Ø primarily used for desalination Ø also removes organic materials, bacteria, viruses, and protozoa
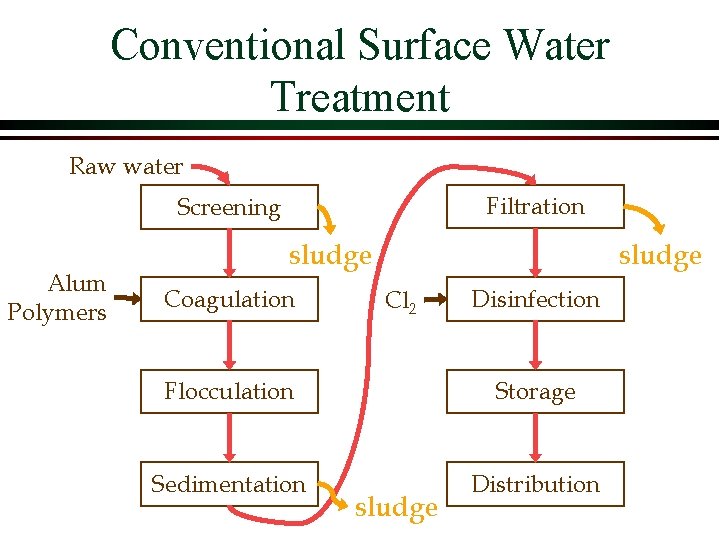
Conventional Surface Water Treatment Raw water Filtration Screening Alum Polymers sludge Coagulation sludge Cl 2 Disinfection Flocculation Storage Sedimentation Distribution sludge

Summary
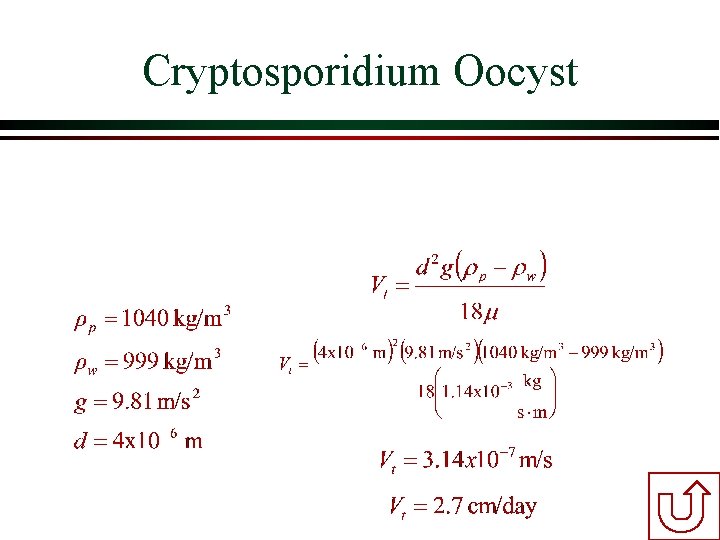
Cryptosporidium Oocyst
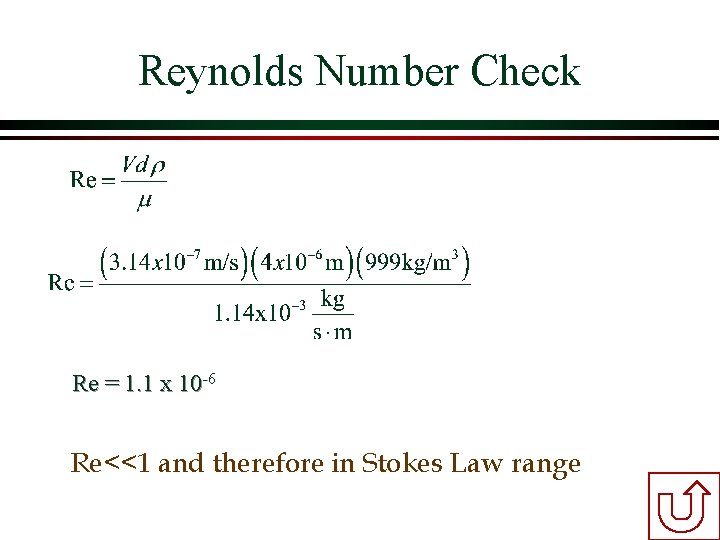
Reynolds Number Check Re = 1. 1 x 10 -6 Re<<1 and therefore in Stokes Law range
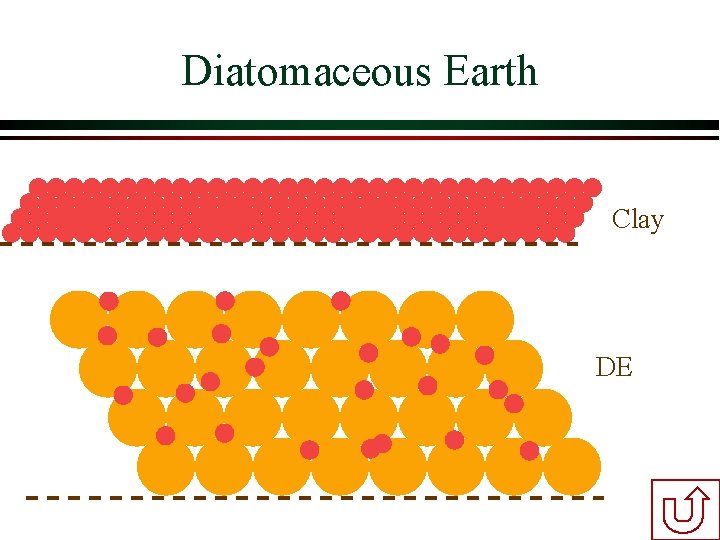
Diatomaceous Earth Clay DE
- Slides: 80