Water Treatment and analysis Water Treatment Average household












- Slides: 24

Water Treatment and analysis

Water Treatment • Average household uses 300 litres per day Criteria – Colourless – Odourless – Safe to drink (no active bacteria) – Fluorinated To ensure water is of an adequate standard for drinking, it must be treated

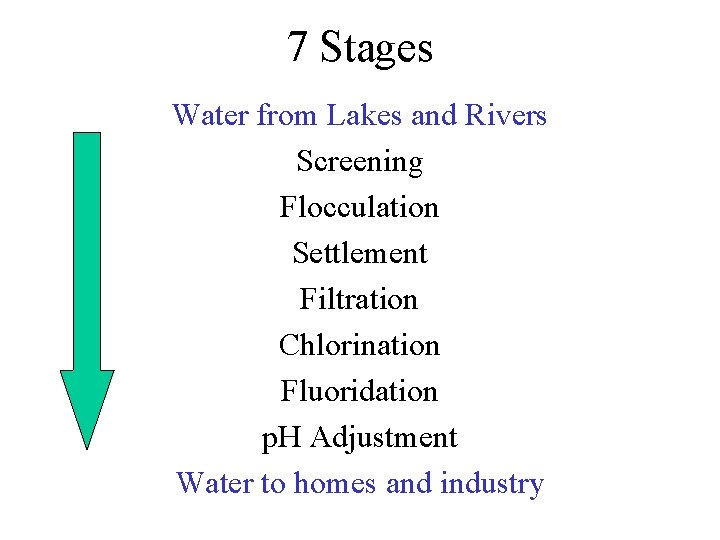
7 Stages Water from Lakes and Rivers Screening Flocculation Settlement Filtration Chlorination Fluoridation p. H Adjustment Water to homes and industry


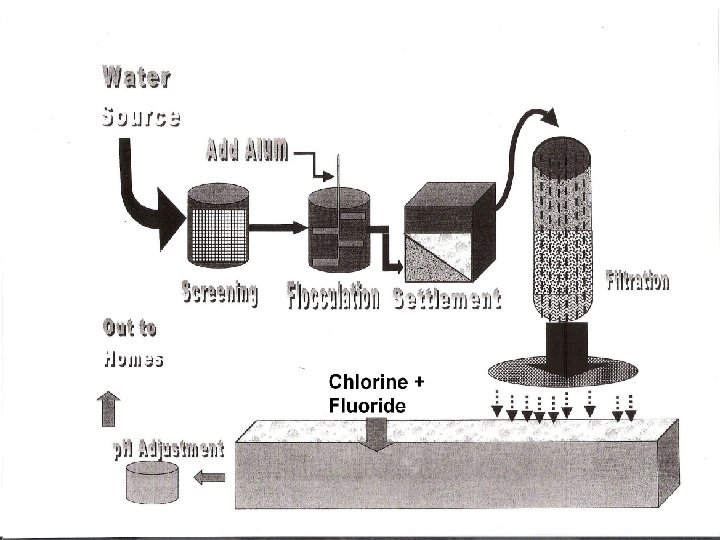
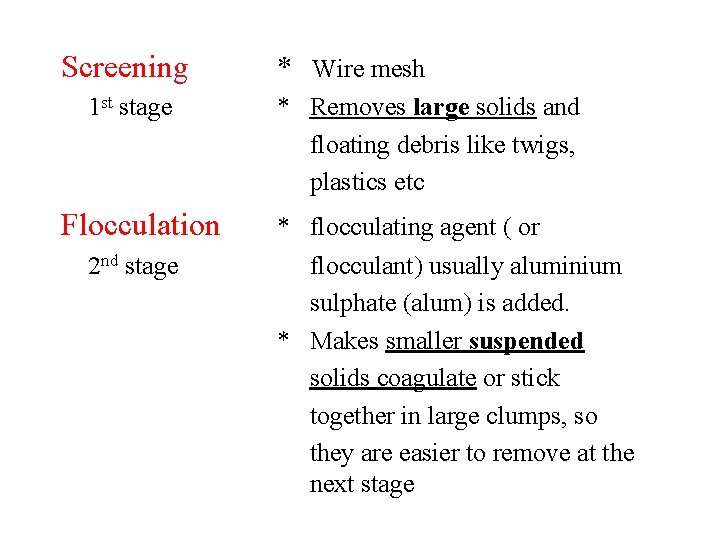
Screening 1 st stage Flocculation 2 nd stage * Wire mesh * Removes large solids and floating debris like twigs, plastics etc * flocculating agent ( or flocculant) usually aluminium sulphate (alum) is added. * Makes smaller suspended solids coagulate or stick together in large clumps, so they are easier to remove at the next stage

Settlement * Large tanks 3 rd stage * Water goes in at the bottom and rises slowly to the top, at < 2 m/hr * Particles settle to the bottom * Over 90% of suspended solids removed at this stage Filtration * Large beds of sand 4 th stage * Removes remaining susp solids * Sand supported on a layer of gravel * Sand cleaned regularly * Water now clear but may contain harmful bacteria

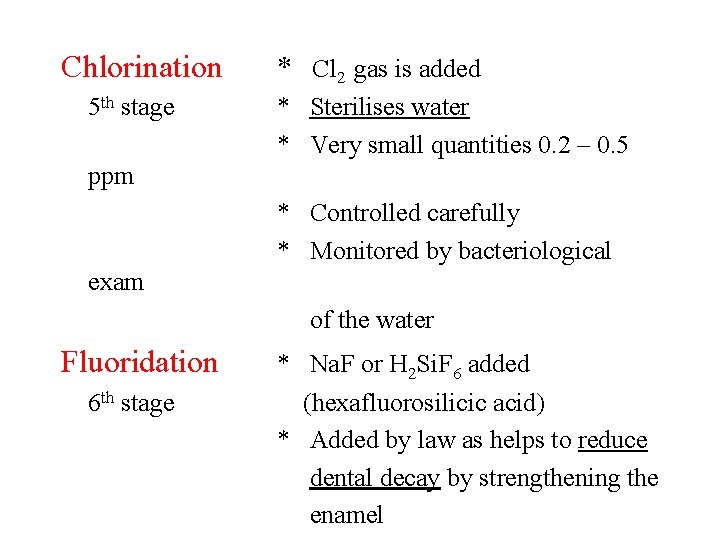
Chlorination 5 th stage * Cl 2 gas is added * Sterilises water * Very small quantities 0. 2 – 0. 5 ppm * Controlled carefully * Monitored by bacteriological exam of the water Fluoridation 6 th stage * Na. F or H 2 Si. F 6 added (hexafluorosilicic acid) * Added by law as helps to reduce dental decay by strengthening the enamel

p. H Adjustment 7 th stage • Optimum level is between 7 - 9 • Too Acidic * may cause damage to pipes addition (lime)to raise the p. H * may be corrected by of Ca(OH)2 * If very hard water, might be softened by addition of Na. CO 3 which is a base • Too Basic * may be corrected by

Oxygen • Dissolves from air into water • Low solubility in water • Temperature inversely affects solubility Temp E. g. Solubility of O 2 11. 3 mg/L @ 10 o. C 9. 2 mg/L @ 20 o. C

Water pollution • The release of substances into the environment that damage the environment is called pollution • 3 main types – Eutrophication – Organic Waste – Heavy metals

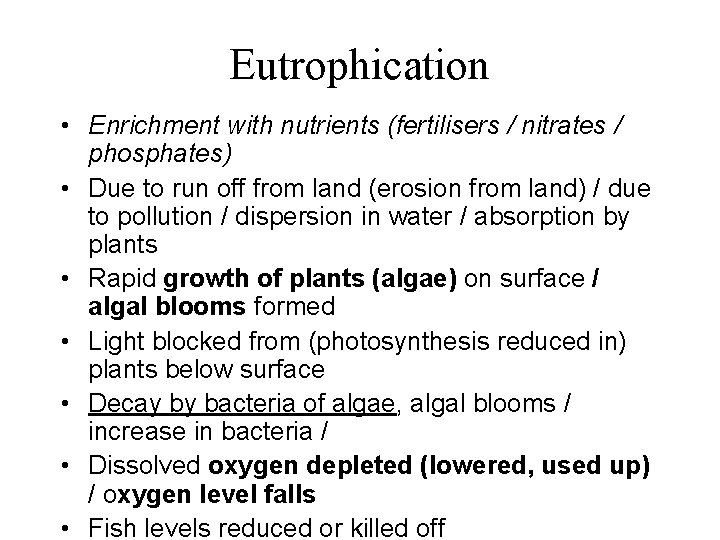
Eutrophication • Enrichment with nutrients (fertilisers / nitrates / phosphates) • Due to run off from land (erosion from land) / due to pollution / dispersion in water / absorption by plants • Rapid growth of plants (algae) on surface / algal blooms formed • Light blocked from (photosynthesis reduced in) plants below surface • Decay by bacteria of algae, algal blooms / increase in bacteria / • Dissolved oxygen depleted (lowered, used up) / oxygen level falls • Fish levels reduced or killed off

Discharge of Organic Waste • Domestic sewage, slurry, silage effluent, effluent from food processing factories, milk, industrial waste, etc • Bacteria and other micro-organisms feed • Waste is broken down, O 2 used up Organic matter + O 2 CO 2 + H 2 O • O 2 used up, reducing levels of fish and possibly killing off fish life. • O 2 gone, anaerobic bacteria take over, river will smell due to presence of by product, Hydrogen Sulphide, H 2 S

Heavy Metals • Lead Pb 2+ • • Cadmium Cd 2+ Mercury Hg 2+ Cumulative poisons Industrial effluents Battery dumping Regulations have brought about improvements • Removed by precipitation

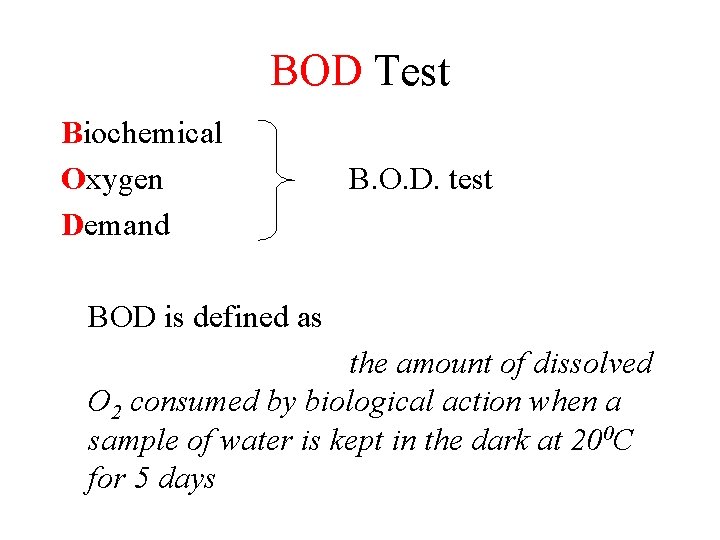
BOD Test Biochemical Oxygen Demand B. O. D. test BOD is defined as the amount of dissolved O 2 consumed by biological action when a sample of water is kept in the dark at 200 C for 5 days

The Winkler method is used to determine the amount of dissolved Oxygen in a water sample 2 bottles sampled • 1 st one tested straight away >> ppm of O 2 • 2 nd one tested after 5 days >> ppm of O 2 • Difference between the two readings is the BOD level, as this is the amount of O 2 used up.

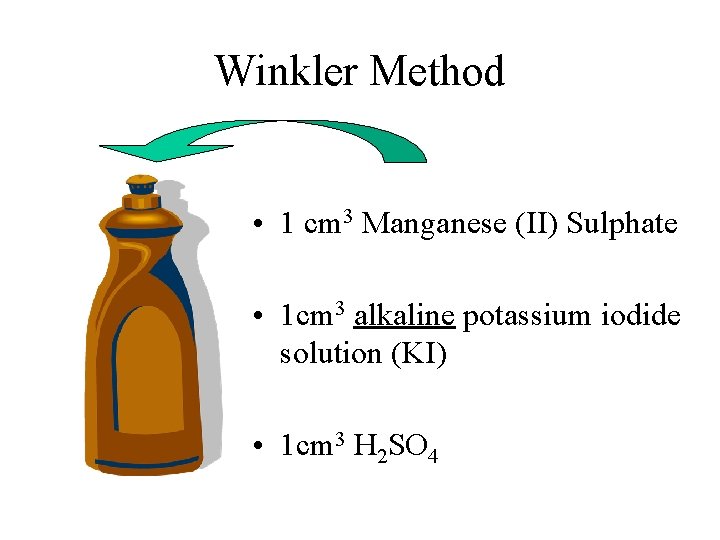
Winkler Method • 1 cm 3 Manganese (II) Sulphate • 1 cm 3 alkaline potassium iodide solution (KI) • 1 cm 3 H 2 SO 4

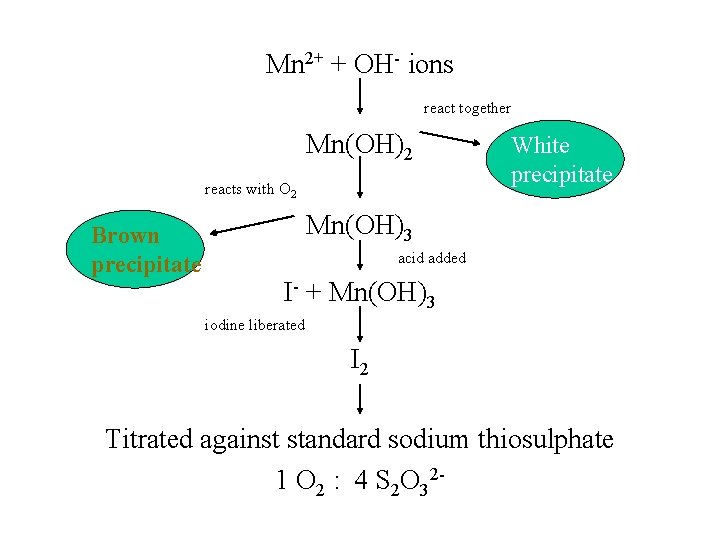
Mn 2+ + OH- ions react together Mn(OH)2 reacts with O 2 Brown precipitate White precipitate Mn(OH)3 acid added I- + Mn(OH)3 iodine liberated I 2 Titrated against standard sodium thiosulphate 1 O 2 : 4 S 2 O 32 -

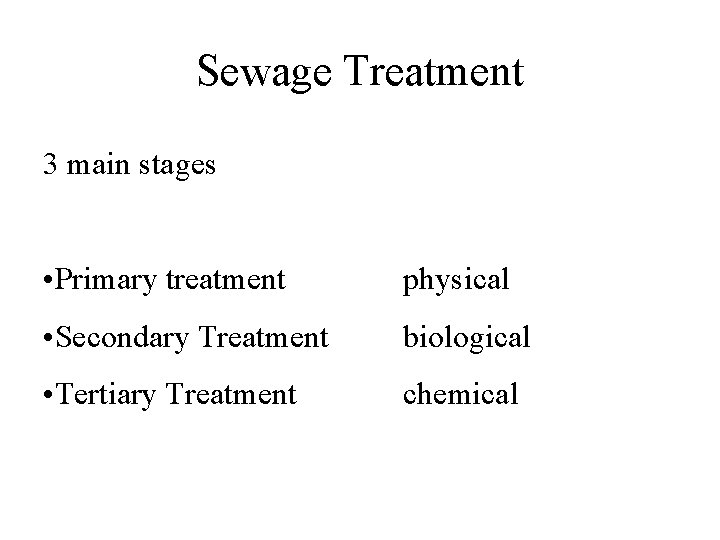
Sewage Treatment 3 main stages • Primary treatment physical • Secondary Treatment biological • Tertiary Treatment chemical


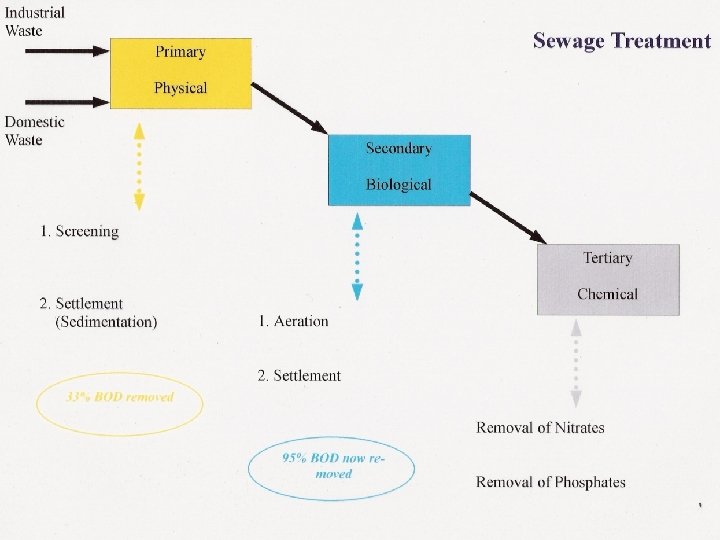
Primary Treatment The physical stage 1. The incoming sewage is screened to remove debris and non-biodegradable material. 2. It passes into large deep sedimentation (settlement) tanks where about 50% of the suspended solids and about 33% of BOD are removed 3. Liquid on top is then passed onto to secondary treatment

Secondary Treatment The biological stage 1. The incoming sewage from first stage of treatment is passed into aeration tanks where mechanical stirrers aerate it. 2. This allows aerobic bacteria and other microorganisms to decompose the solid matter into a harmless sludge called Activated Sludge. 3. Sewage then flows into settling tanks, sludge can be reused, or or recyled to make methane 4. About 95% of BOD removed

Tertiary Treatment The chemical stage 1. When the liquid passes out of the second round of settling, it is clean and inoffensive, but it may contain compounds of nitrates and phosphates 2. Phosphates from washing powders and washing up liquids, and nitrates from organic material in sewage 3. Must be removed before water discharged into river as could cause eutrophication.

1. Phosphate removal • aluminium sulphate added, aluminium phosphate precipitates out • Iron (III) chloride added, iron (III) phosphate precipitates out • Lime may also be used • Insoluble phosphate compounds allowed to settle out before discharge into waterway 2. Nitrate removal • difficult and expensive as nitrogen may be present in many forms, NH 3, NO 2 - , NO 3 -, organic compounds containing N

Instrumental Methods of Analysis • p. H meter • Atomic Absorption Spectrometry • Colorimetry – determining conc. of free chlorine in swimming pool water – DPD tablets added, reacts with chlorine in water – Compare colour with ones of known concentration – Comparator - disc of precalibrated solutions Or – Colorimeter – measure absorbance of known solutions and plot graph
 Water and water and water water
Water and water and water water Second equation of motion
Second equation of motion Useful and harmful materials worksheets
Useful and harmful materials worksheets Firm and household
Firm and household Family and household transition
Family and household transition Household behavior and consumer choice
Household behavior and consumer choice Household metric system
Household metric system Survey of household economics and decisionmaking
Survey of household economics and decisionmaking Microbes in household products
Microbes in household products Rhonda dalton
Rhonda dalton Household conversions for nursing
Household conversions for nursing What's household composition
What's household composition Safety symbols on household products
Safety symbols on household products Series circuit questions
Series circuit questions 5 properties of bases
5 properties of bases Common household chores method of heat transfer
Common household chores method of heat transfer English and metric system
English and metric system Head of household discipline
Head of household discipline Pharmaceutical measurements
Pharmaceutical measurements International household survey network
International household survey network Snc set symbol
Snc set symbol How does nitrogen help plants
How does nitrogen help plants Household hardware
Household hardware Saints in caesar's household
Saints in caesar's household Household hardware
Household hardware