Water The Universal Solvent SOL BIO 3 a

Water The Universal Solvent SOL BIO 3 a

SOL BIO 3 a OBJECTIVE: TSW understand the chemical and biochemical principles essential for life. Key concepts include- • water chemistry and its impact on life processes.
Review • Elements are made up of atoms • Compounds are made up of molecules.

• A molecule is so small that there are billions of molecules in a single drop of water. About 60 million water molecules could be stretched side by side across a penny.

• Water is the ONLY compound that commonly exists in all 3 phases (solid, liquid, gas) on Earth. There would be no life on Earth without water.
• About 2/3 of the mass of a cell is made up of water, and most of the biochemical processes of life occur in water solutions.

SOLVENT- the substance that does the dissolving. (liquid) SOLUTE- the substance that is dissolved. (solid) A large number of substances will dissolve in water. Water is the universal solvent.
• Therefore, the water inside and outside of cells is able to carry nutrients into and around cells and wastes away from cells.

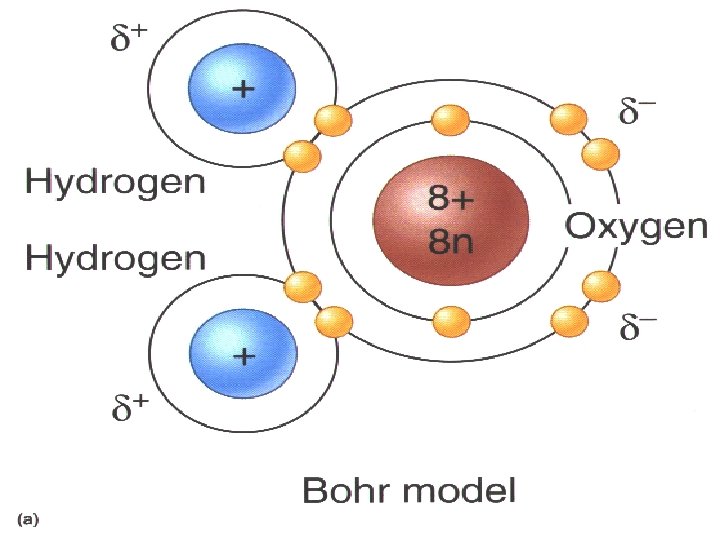
Water is a POLAR molecule Polarity - the attraction of positive and negative electrical charges OPPOSITES ATTRACT

Adhesion: the tendency of two DIFFERENT substances to stick together, like water molecules stick to glass.

Cohesion: the tendency of two SIMILAR substances to stick together.

Capillary Action: the tendency of a liquid substance to move along the surface of a solid substance due to adhesion (as in water climbing a glass tube or inside a tree), even in spite of gravitational or other forces acting in the opposite direction
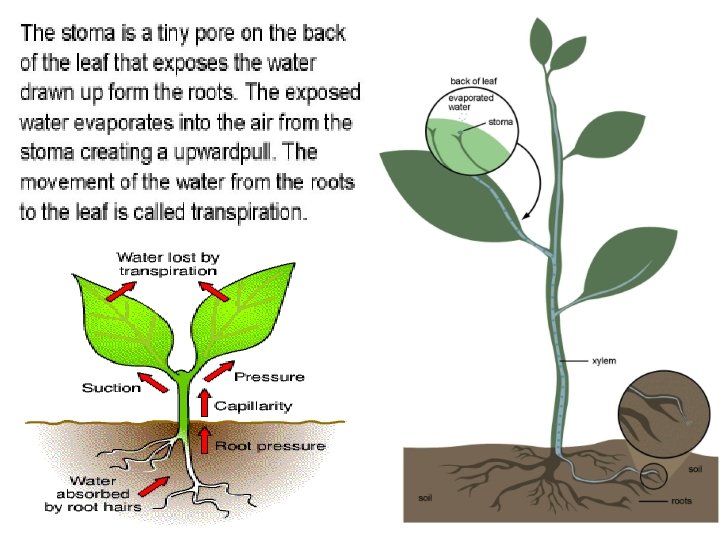
Transpirati on

• Hydrolysis- a chemical reaction where water is involved. • Hydrophilic- “water loving” can be dissolved in water, polar substances. • Hydrophobic- “water hating” cannot be dissolved in water, non-polar substances, Ex. fat, oil, soap Like Dissolves Like
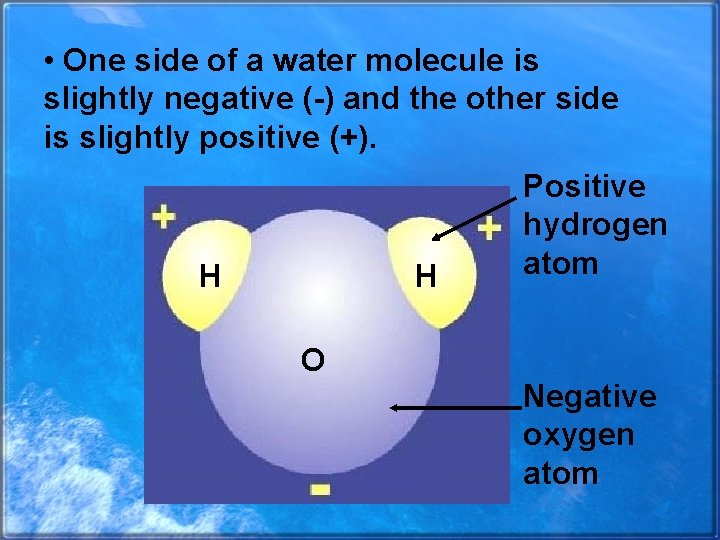
• One side of a water molecule is slightly negative (-) and the other side is slightly positive (+). H H Positive hydrogen atom O Negative oxygen atom
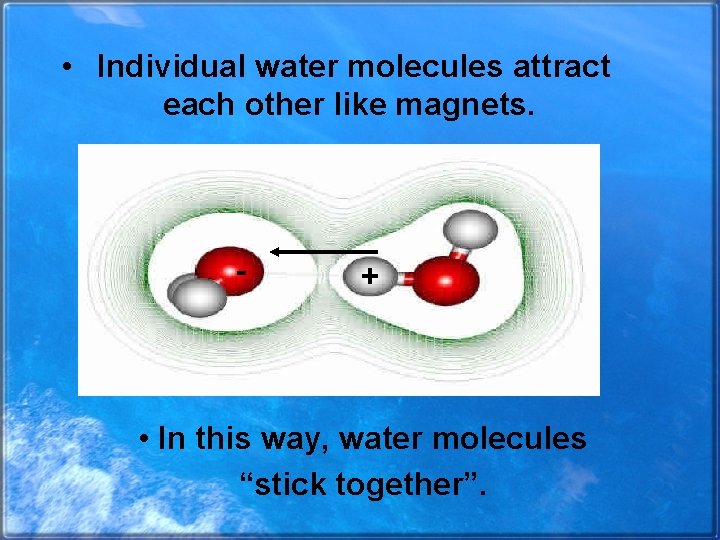
• Individual water molecules attract each other like magnets. - + • In this way, water molecules “stick together”.

Resistance to Temperature Change • Water is able to absorb a large amount of heat energy before it changes temperature.

• Water is a liquid from 0 ºC to 100 ºC

• Water absorbs heat when it evaporates, allowing organisms to release excess heat.

• Large bodies of water can affect the climate. - As a result, lakes and oceans stabilize air and land temperatures.

High Surface Tension • Certain insects can walk on water because of its high surface tension. • Polarity gives water its high surface tension. • Ex. Water strider

Solid expansion For most substances, solids are more dense than liquids. But the special properties of water make it less dense as a solid - ice floats on water!

When water freezes, the water molecules line up, and as they do, they move farther apart !
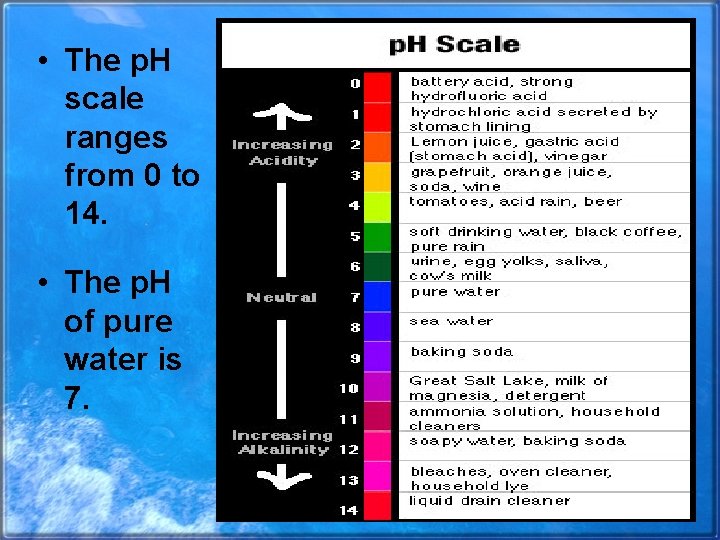
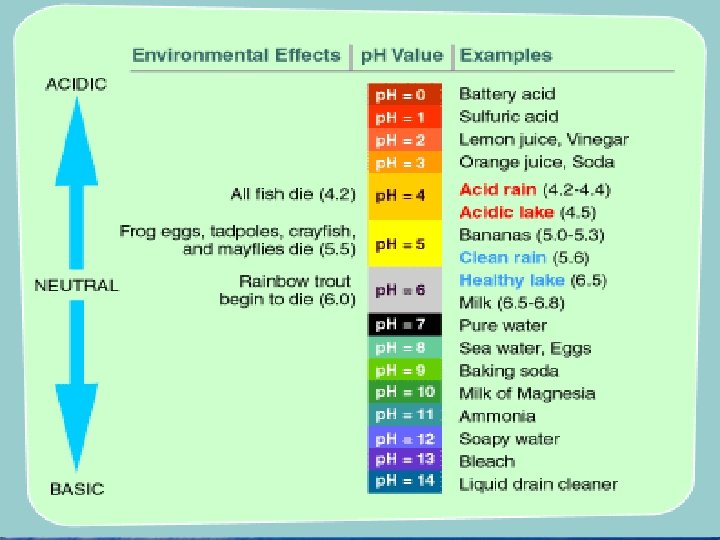
• The p. H scale ranges from 0 to 14. • The p. H of pure water is 7.

• Substances added to water can lower or raise the p. H. • A solution with a p. H below 7 is acidic. • A solution with a p. H above 7 is basic.

• Organisms can tolerate only small changes in p. H because every cell has a particular p. H at which it functions best. • For example, changes in p. H cause changes in the shapes of enzymes, resulting in a change in their activity.

Public Health • In the past, streams and rivers were often used to dispose of human waste. • These were called open sewers.

• This led to disease and contamination of drinking water. • Advances in water treatment and sanitary sewers have helped to eliminate diseases from human waste.

Humans can live without food for more than two months, but cannot live for more than a week without water. No =

• Clean, fresh water is essential for life. • We must protect our water sources from pollution!!!!
- Slides: 32