WATER TAP WATER WATER THAT COMES FROM THE



















- Slides: 31

WATER

TAP WATER • WATER THAT COMES FROM THE MAIN SUPPLY OF THE LOCAL WATER SYSTEM • HUDSON GETS ITS TAP WATER FROM DIFFERENT WATER DEPARTMENTS IN CITIES SUCH AS AKRON, CLEVELAND, STOW • HTTP: //WWW. HUDSON. OH. US/INDEX. ASPX? NID=127

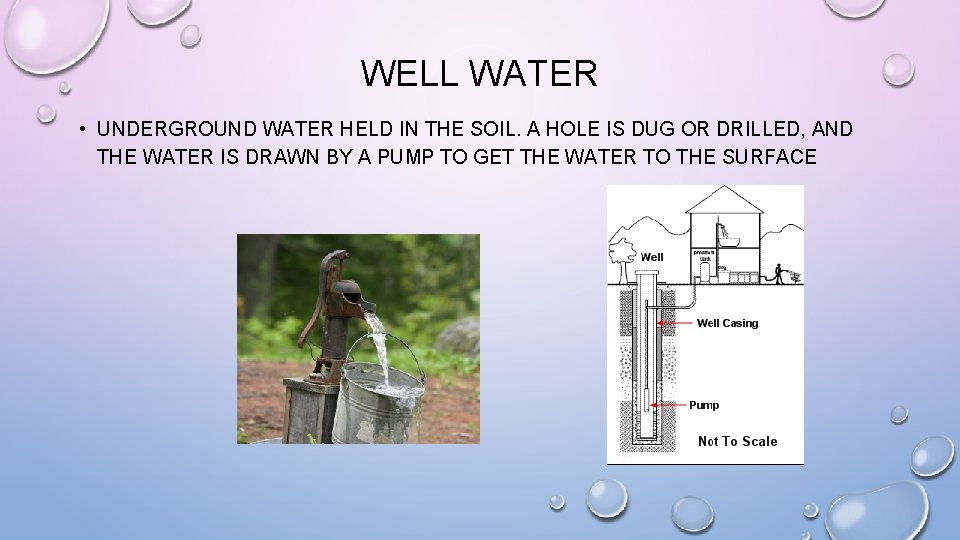
WELL WATER • UNDERGROUND WATER HELD IN THE SOIL. A HOLE IS DUG OR DRILLED, AND THE WATER IS DRAWN BY A PUMP TO GET THE WATER TO THE SURFACE

DISTILLED WATER • WATER THAT IS PURIFIED BY BOILING THE WATER, AND AS THE WATER IS BOILED IT TURNS TO STEAM • THE PROCESS OF BOILING THE WATER AND CONDENSING THE STEAM CAUSES THE WATER TO LEAVE BEHIND ITS CONTAMINANTS, OR “DISSOLVED SOLIDS. ”

SPRING WATER • WATER OBTAINED FROM AN UNDERGROUND SOURCE THAT FLOWS NATURALLY TO EARTH’S SURFACE THROUGH A SPRING

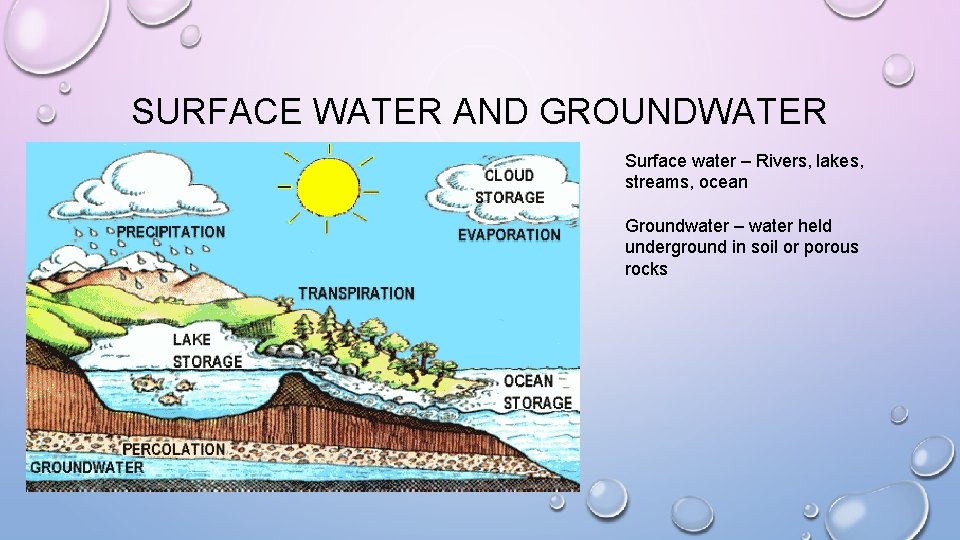
SURFACE WATER AND GROUNDWATER Surface water – Rivers, lakes, streams, ocean Groundwater – water held underground in soil or porous rocks

CONTAMINATION • Any substances picked up by water • Can be helpful or harmful • Pollution from human activities can be harmful and cause contaminants to enter water supply • Contaminants may be biological or chemical • Biological – contaminants from living or once living things that may cause disease, such as viruses, parasites, or bacteria • Chemical – non-living substances such as fertilizers, gasoline, oil, heavy metals

• HTTP: //WWW. NBCNEWS. COM/NEWS/US-NEWS/AFTER-SPILL-MONTANA-TOWNAWAITS-WORD-WATER-SUPPLY-N 291756

EPIDEMIC • When many people in a population or community are affected with a disease at the same time • Examples: ebola, bubonic plague, smallpox, influenza, HIV, H 1 N 1, SARS, measles

QUALITATIVE VS QUANTITATIVE • Qualitative: describes characteristics that we can perceive without making measurements • Examples: colors, how somethings feels, smells, tastes, etc. • Quantitative: information that can be measured using numbers • Examples: mass, volume, number of pennies, length, etc.

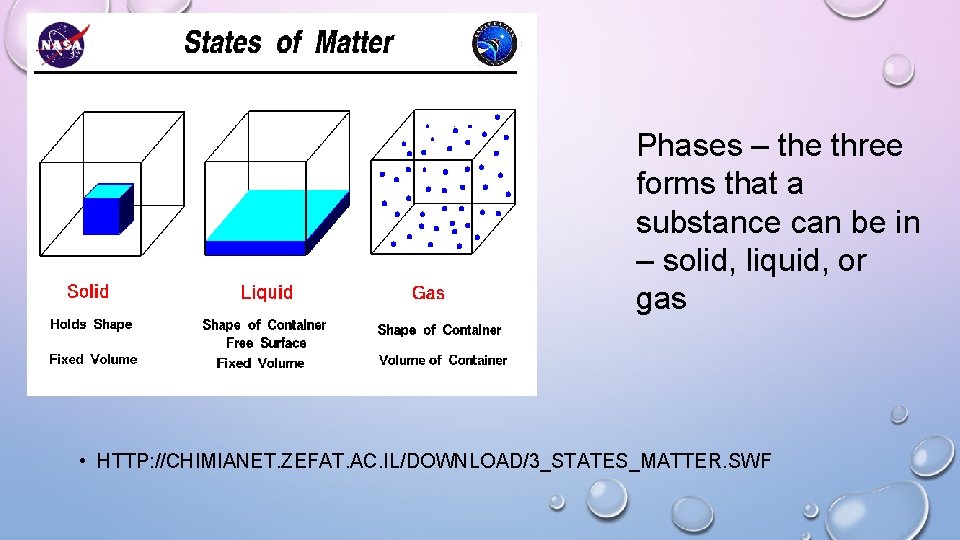
Phases – the three forms that a substance can be in – solid, liquid, or gas • HTTP: //CHIMIANET. ZEFAT. AC. IL/DOWNLOAD/3_STATES_MATTER. SWF

DENSITY • Density – mass per unit volume • Density = mass/volume • A substance has a mass of 14 g and a volume of 20 m. L. What is its density? • 14 g / 20 m. L = 0. 7 g m/L • What is more dense? A piece of silver with a mass of 250 g or a piece of silver with a mass of 700 g? • Both pieces of silver have the same density! • This is because density is a physical property of substances. It does not change depending on how much you have. We can use densities to identify unknown substances

• ATOM – • Smallest particle of all matter • The name atom comes from a greek word which means that which can’t be cut

• ELEMENT – • Made of only one kind of atom • Can not be broken down by ordinary chemical means

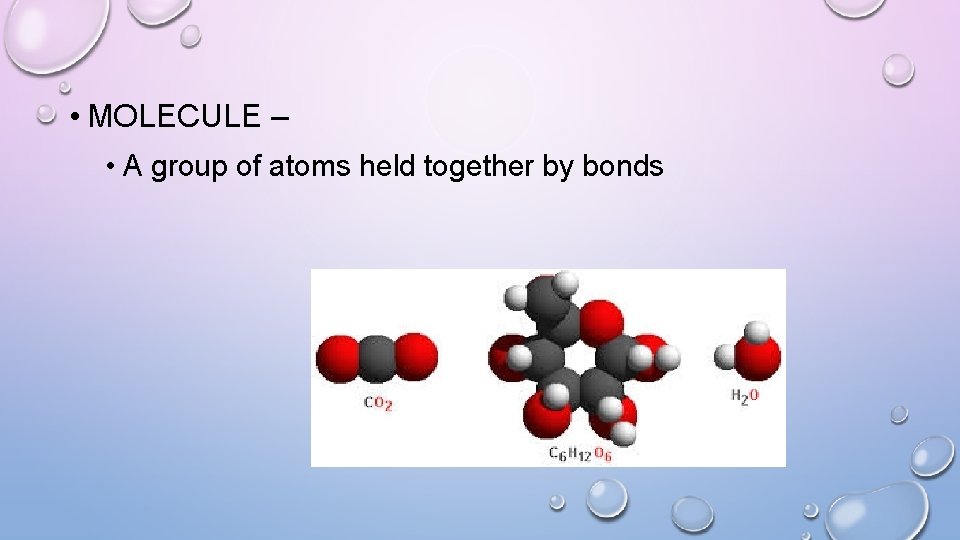
• MOLECULE – • A group of atoms held together by bonds

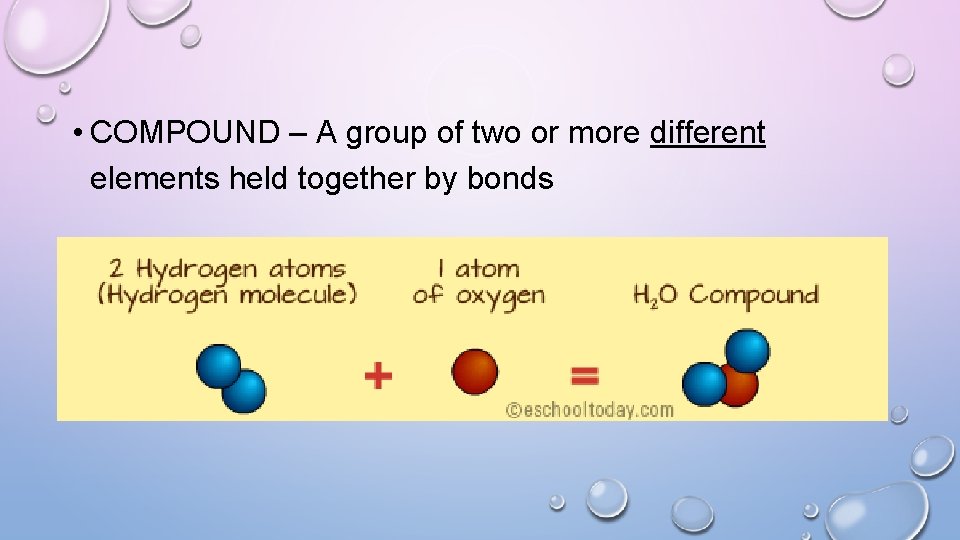
• COMPOUND – A group of two or more different elements held together by bonds

ICE CREAM ANALOGY • YOU GO TO AN ICE CREAM SHOP • THEY HAVE 30 FLAVORS OF ICE CREAM • THE 30 FLAVORS REPRESENT THE DIFFERENT ELEMENTS • THE SMALLEST AMOUNT THAT THE STORE WILL SELL IS A SCOOP • THIS IS AN ATOM • I CAN PUT TWO OR MORE SCOOPS OF ICE CREAM TOGETHER • THIS IS A MOLECULE • THE TWO SCOOPS I PUT TOGETHER ARE TWO DIFFERENT FLAVORS • THIS IS A COMPOUND

TRUE OR FALSE • A compound is always a molecule • True • A molecule is always a compound • False

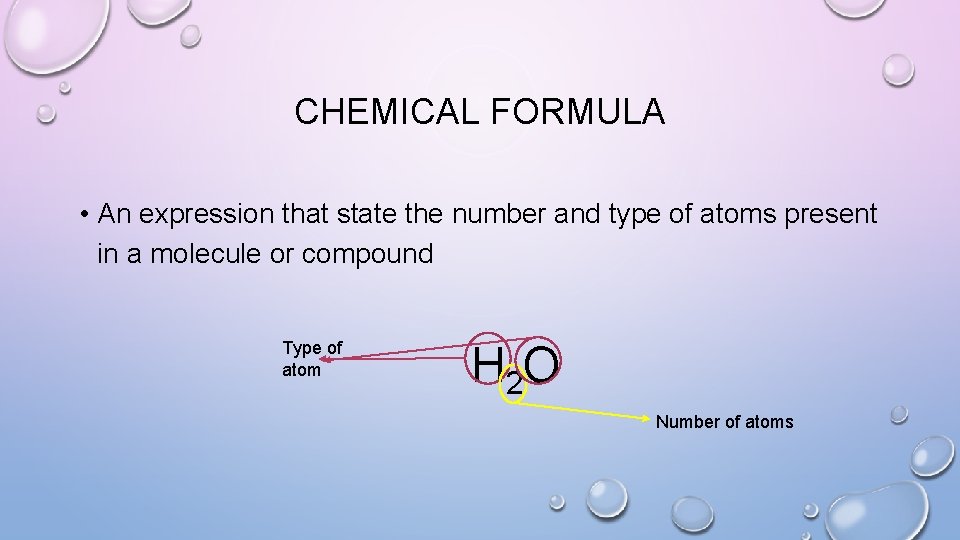
CHEMICAL FORMULA • An expression that state the number and type of atoms present in a molecule or compound Type of atom H 2 O Number of atoms

ACTIVITY 37: WHAT DISSOLVES VOCABULARY • Soluble – when a substance is able to be dissolved • Example: sugar, salt • Solvent – a substance that has the ability to dissolve other substances • Example: water • Solution – a liquid mixture that is composed of a solute and solvent • Example: salt water, sugar water

SOLUTIONS • When a substance mixed completely in the water, dissolves, disappears, and becomes clear, it is a solution • Examples: sodium chloride (cups 1 and 2), copper chloride (cups 3 and 4) • The sodium chloride and copper chloride are soluble in water (able to be dissolved) • The cornstarch and iron chloride would be ____ (not able to be dissolved) • Answer: insoluble

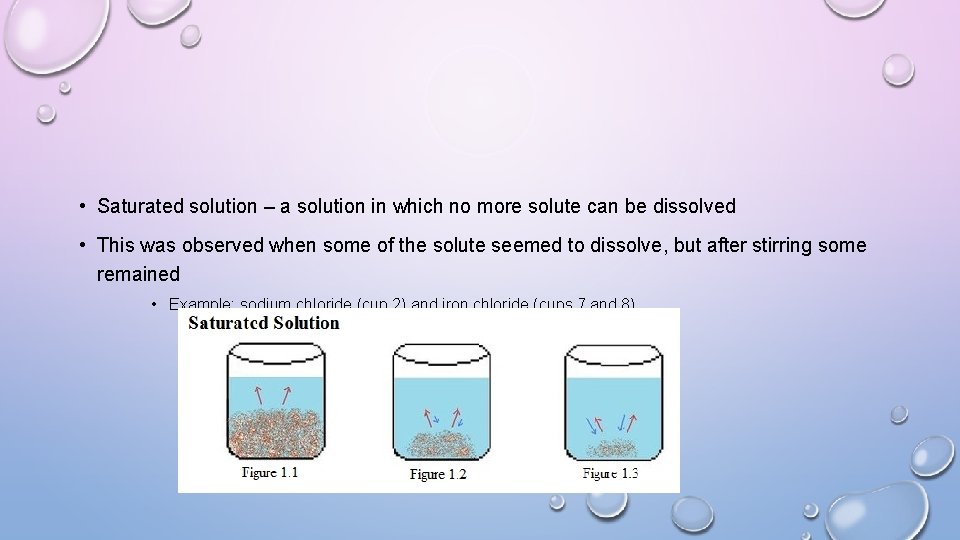
• Saturated solution – a solution in which no more solute can be dissolved • This was observed when some of the solute seemed to dissolve, but after stirring some remained • Example: sodium chloride (cup 2) and iron chloride (cups 7 and 8)

ACTIVITY 40: PARTS PER MILLION VOCABULARY • Concentration - amount of solute in a solution • Dilute – making a liquid weaker by adding more solvent • Example: adding water to pure orange juice • Concentrated Solution – a solution that contains a large amount of solute • Soft drinks (have a lot of sugar dissolved in water)

SERIAL DILUTION • If I have a 10% solution, we know that the fraction to represent this concentration is 1/10 (10/100 = 1/10) • We know that for a serial dilution, we dilute by a factor of 10. • So, cup one contains the 1/10, or 10% solution. • Cup two would contain (1/10) * (1/10) or 1/100 • Cup three would contain (1/100) * (1/10) or (1/1, 000) • Cup four would contain (1/1, 000) * (1/10) or (1/10, 000) • …. And so on

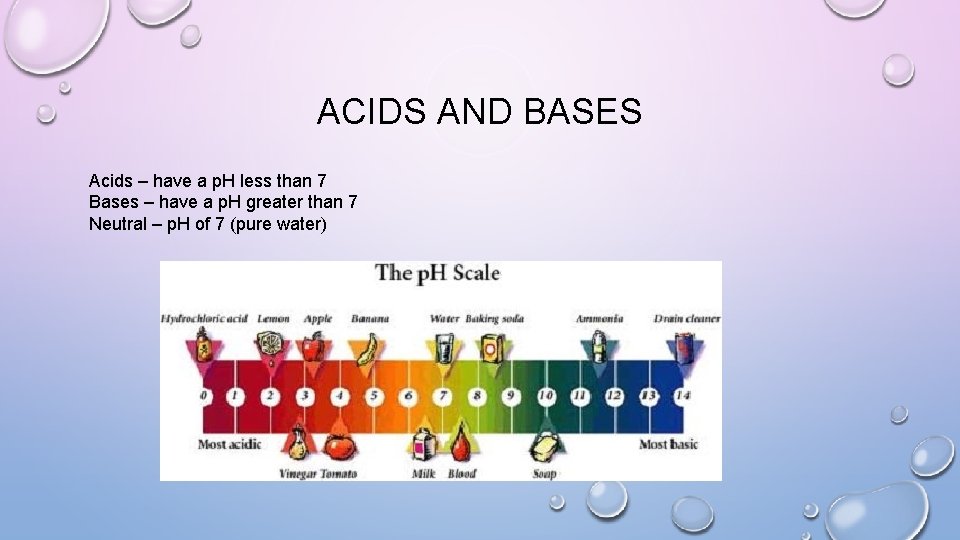
ACIDS AND BASES Acids – have a p. H less than 7 Bases – have a p. H greater than 7 Neutral – p. H of 7 (pure water)

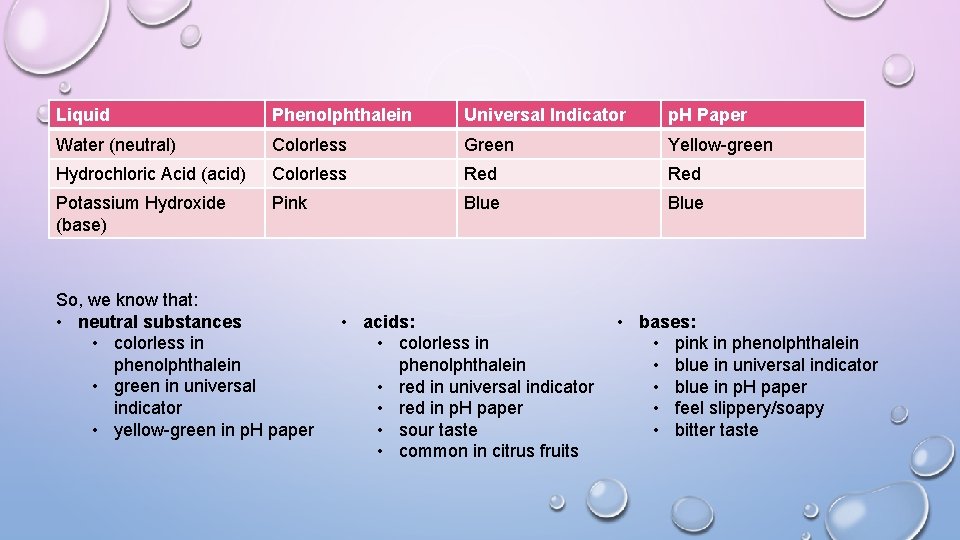
Liquid Phenolphthalein Universal Indicator p. H Paper Water (neutral) Colorless Green Yellow-green Hydrochloric Acid (acid) Colorless Red Potassium Hydroxide (base) Pink Blue So, we know that: • neutral substances • colorless in phenolphthalein • green in universal indicator • yellow-green in p. H paper • acids: • colorless in phenolphthalein • red in universal indicator • red in p. H paper • sour taste • common in citrus fruits • bases: • pink in phenolphthalein • blue in universal indicator • blue in p. H paper • feel slippery/soapy • bitter taste

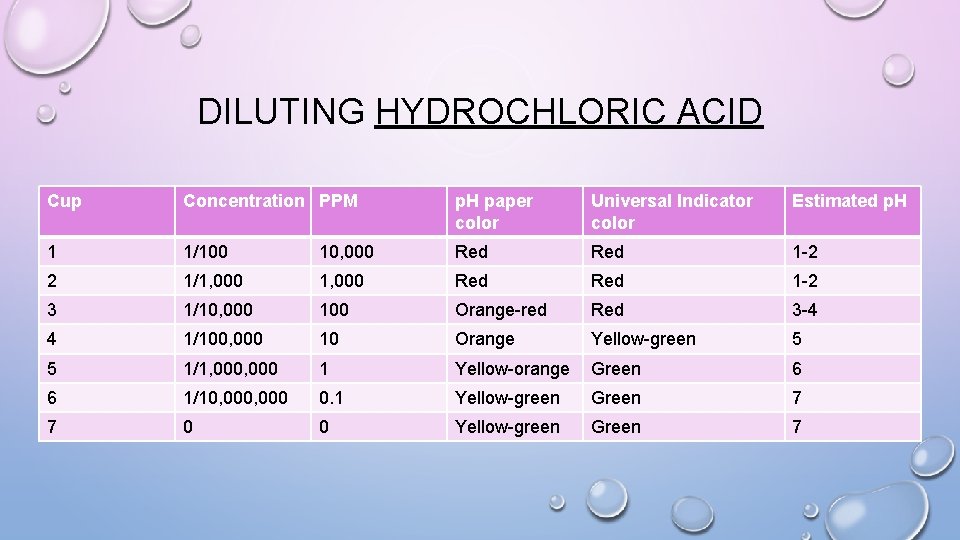
DILUTING HYDROCHLORIC ACID Cup Concentration PPM p. H paper color Universal Indicator color Estimated p. H 1 1/100 10, 000 Red 1 -2 2 1/1, 000 Red 1 -2 3 1/10, 000 100 Orange-red Red 3 -4 4 1/100, 000 10 Orange Yellow-green 5 5 1/1, 000 1 Yellow-orange Green 6 6 1/10, 000 0. 1 Yellow-green Green 7 7 0 0 Yellow-green Green 7

DILUTING POTASSIUM HYDROXIDE Cup Concentration PPM p. H paper color Universal Indicator color Estimated p. H 1 1/100 10, 000 Deep blue Blue 10 -11 2 1/1, 000 Deep blue Blue 10 3 1/10, 000 100 Blue-green Blue 9 4 1/100, 000 10 Pale green Blue-green 8 5 1/1, 000 1 Yellow-green Blue-green 7 6 1/10, 000 0. 1 Yellow-green Green 7 7 0 0 Yellow-green Green 7

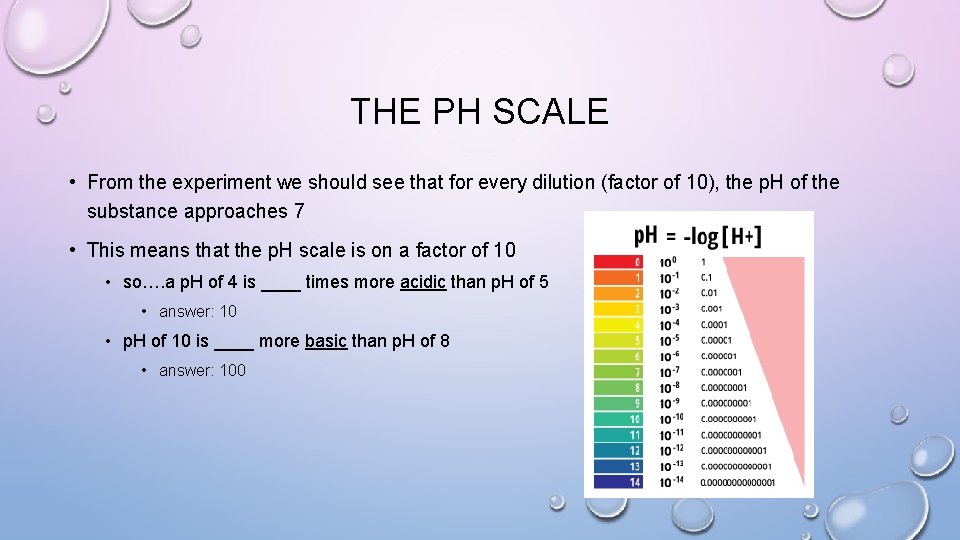
THE PH SCALE • From the experiment we should see that for every dilution (factor of 10), the p. H of the substance approaches 7 • This means that the p. H scale is on a factor of 10 • so…. a p. H of 4 is ____ times more acidic than p. H of 5 • answer: 10 • p. H of 10 is ____ more basic than p. H of 8 • answer: 100

WATER TREATMENT 1. Coagulation • Involves two steps: • Flocculation – chemicals put into the water to attract contaminants • Sedimentation – letting the clumps formed by flocculation to sink to the bottom 2. Filtration – removes solid contaminants from the water 3. Disinfection – chemicals added to water to kill biological contaminants 4. Fluoridation – fluoride added to water to reduce tooth decay and cavities

READING A WATER QUALITY REPORT • MCL – MAXIMUM CONTAMINANT LEVEL • IF SOMETHING HAS A MCL OF 200 PPM, THAT MEANS ANYTHING OVER 200 PPM IS CONSIDERED UNSAFE • AL – ACTION LEVEL • CONCENTRATION IN WHICH THE WATER SUPPLIER MUST TREAT THE WATER OR TAKE OTHER APPROPRIATE MEASURES
 Twolittlehands
Twolittlehands Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block xoang nhĩ là gì
Block xoang nhĩ là gì Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thể thơ truyền thống
Thể thơ truyền thống Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã First comes love then comes marriage
First comes love then comes marriage Tap water homogeneous or heterogeneous
Tap water homogeneous or heterogeneous Is tap water a heterogeneous mixture
Is tap water a heterogeneous mixture Variables en un experimento
Variables en un experimento Tap water
Tap water Water and water and water water
Water and water and water water Bổ thể
Bổ thể Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Hát lên người ơi
Hát lên người ơi Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là điện thế nghỉ
điện thế nghỉ Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Phép trừ bù
Phép trừ bù Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh