WATER SOFTENING l removal of hardness Hardness is







- Slides: 12

WATER SOFTENING l removal of hardness » Hardness is? . . . primarily Ca, Mg, plus Fe, Mn, St, Al l How is Softening done? . . . Precipitation of Ca and Mg, or Ion exchange of Ca / Mg with ion such as Na 1

Why bother? l Hardness in 300 -500 mg/l as Ca. CO 3 range considered excessive high soap consumption scaling in heating vessels and pipes Even > 150 mg/l may result in consumer objection l 60 -120 mg/l as Ca. CO 3 is considered a moderate amount l 2

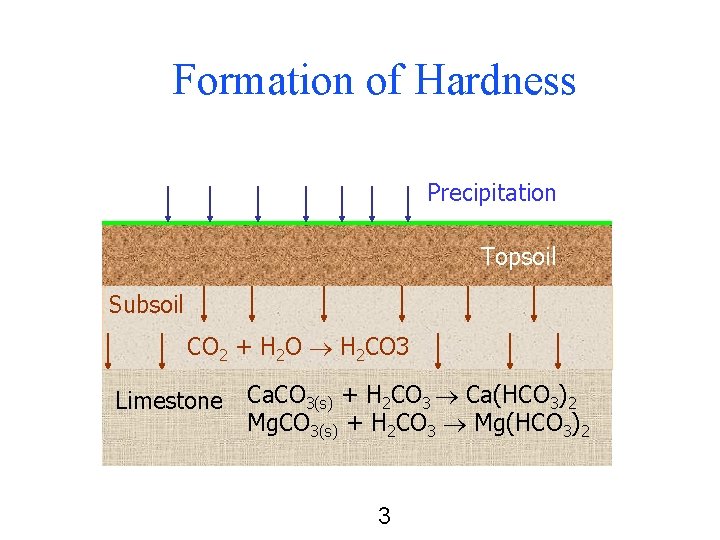
Formation of Hardness Precipitation Topsoil Subsoil CO 2 + H 2 O H 2 CO 3 Limestone Ca. CO 3(s) + H 2 CO 3 Ca(HCO 3)2 Mg. CO 3(s) + H 2 CO 3 Mg(HCO 3)2 3

Hardness l Carbonate Hardness » Often called "temporary hardness" because heating the water will remove it. When the water is heated, the insoluble carbonates will precipitate and tend to form bottom deposits in water heaters. » Ca 2+, Mg 2+ associated with HCO 3 -, CO 32» CH = TH or Total alkalinity, whichever is less 4

Hardness l Non-Carbonate Hardness » Called permanent hardness because it is not removed when the water is heated. It is much more expensive to remove non-carbonate hardness than carbonate hardness. » Ca 2+, Mg 2+ associated with other ions, Cl-, NO 3 -, SO 42» NCH = TH - CH » If Alkalinity Total hardness, then NCH = 0 5

Hardness Units l l milligrams per liter (mg/L) as calcium carbonate (most common) parts per million (ppm) as calcium carbonate grains per gallon of hardness (to convert from grains per gallon to mg/L, multiply by 17. 1) equivalents/liter (eq/L) 6

LIME - SODA ASH SOFTENING Addition of lime, Ca(OH)2, & soda ash, Na 2 CO 3 causes precipitation of Ca, Mg l Lime often added as Ca. O, quick lime l » Ca. O + H 20 --> Ca(OH)2 l Three basic processes » Excess lime treatment » Selective calcium removal » Split treatment 7

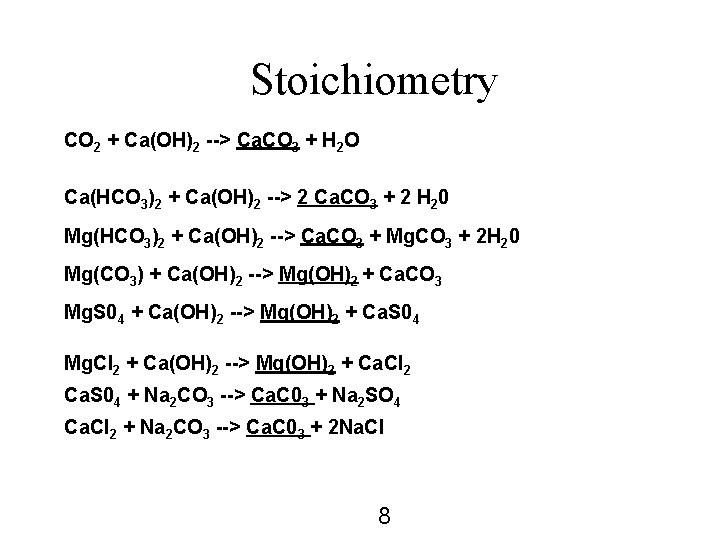
Stoichiometry CO 2 + Ca(OH)2 --> Ca. CO 3 + H 2 O Ca(HCO 3)2 + Ca(OH)2 --> 2 Ca. CO 3 + 2 H 20 Mg(HCO 3)2 + Ca(OH)2 --> Ca. CO 3 + Mg. CO 3 + 2 H 20 Mg(CO 3) + Ca(OH)2 --> Mg(OH)2 + Ca. CO 3 Mg. S 04 + Ca(OH)2 --> Mg(OH)2 + Ca. S 04 Mg. Cl 2 + Ca(OH)2 --> Mg(OH)2 + Ca. Cl 2 Ca. S 04 + Na 2 CO 3 --> Ca. C 03 + Na 2 SO 4 Ca. Cl 2 + Na 2 CO 3 --> Ca. C 03 + 2 Na. Cl 8

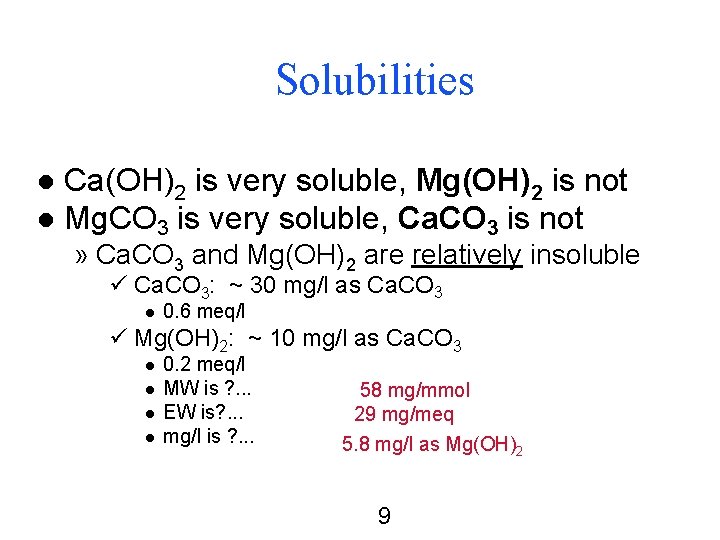
Solubilities Ca(OH)2 is very soluble, Mg(OH)2 is not l Mg. CO 3 is very soluble, Ca. CO 3 is not l » Ca. CO 3 and Mg(OH)2 are relatively insoluble ü Ca. CO 3: ~ 30 mg/l as Ca. CO 3 l 0. 6 meq/l ü Mg(OH)2: ~ 10 mg/l as Ca. CO 3 l l 0. 2 meq/l MW is ? . . . EW is? . . . mg/l is ? . . . 58 mg/mmol 29 mg/meq 5. 8 mg/l as Mg(OH)2 9

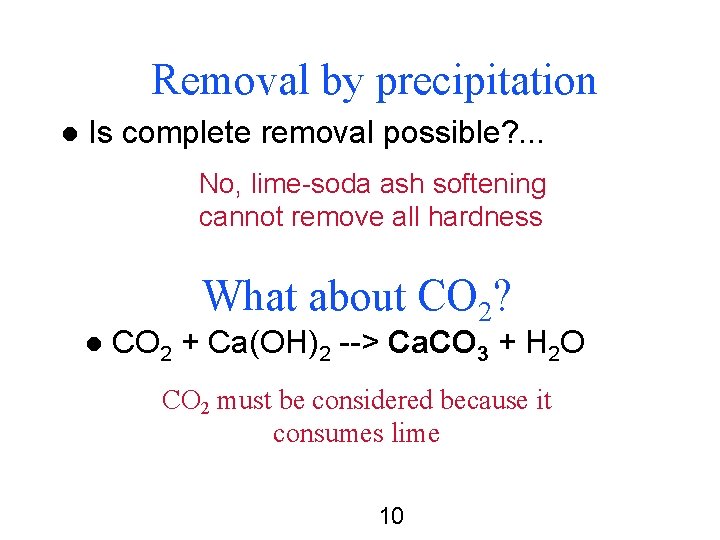
Removal by precipitation l Is complete removal possible? . . . No, lime-soda ash softening cannot remove all hardness What about CO 2? l CO 2 + Ca(OH)2 --> Ca. CO 3 + H 2 O CO 2 must be considered because it consumes lime 10

Effectiveness l 80 -100 mg/l as Ca. CO 3 is usually considered acceptable result of limesoda ash softening, » as long as Mg is < 40 mg/l as Ca. CO 3 üany more causes scaling in heating vessels 11

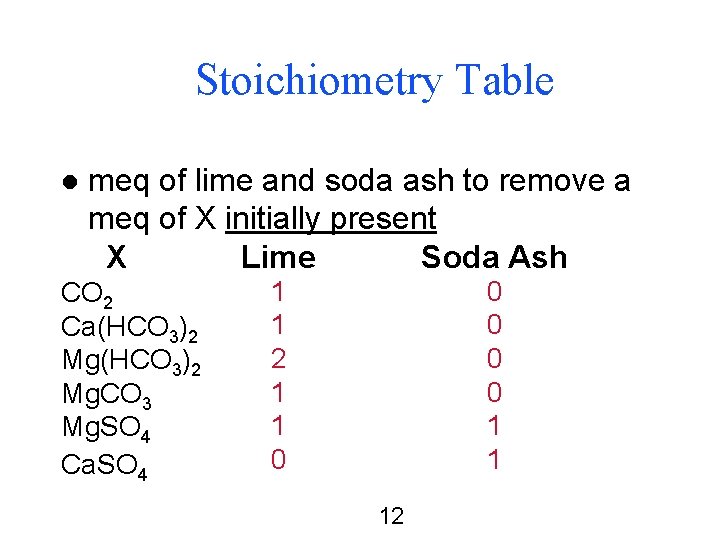
Stoichiometry Table l meq of lime and soda ash to remove a meq of X initially present X Lime Soda Ash CO 2 Ca(HCO 3)2 Mg. CO 3 Mg. SO 4 Ca. SO 4 1 1 2 1 1 0 0 0 1 1 12