Water Quality Testing Water quality is the physical

















- Slides: 20

Water Quality & Testing § Water quality is the physical, chemical and biological characteristics of water § The vast majority of surface water on the planet is neither potable (fit for drinking) nor toxic (poisonous) – essentially, not good or bad § Approximately ___% of the world’s 25 population has no access to potable water

Water Quality § No single test or simple property can tell whether water is polluted or not § “Industrial” pollution is a major cause of water pollution… in addition to: – runoff from agricultural areas – urban storm water runoff – untreated sewage (especially in developing countries)

Water Contamination § Contaminants found in untreated water include: – – Micro-organisms (viruses and bacteria) inorganic contaminants (salts and metals) pesticides and herbicides organic chemical contaminants from industrial processes and petroleum use – radioactive contaminants – sediments § Water quality depends on the local geology and ecosystem, as well as human uses – (sewage dispersion, industrial pollution, use of water bodies as a heat sink)

Just list a few exam Sources of Water Pollution § § § ples… Industrial discharge of chemical wastes and byproducts Discharge of poorly-treated or untreated sewage Surface runoff containing pesticides or fertilizers Deforestation: “slash and burn” farming practice, which is often done to clear land for agriculture – contributing to erosion and run-off Surface runoff containing spilled petroleum products Surface runoff from construction sites, farms, or paved and other impervious surfaces Discharge of contaminated and/or heated water used for industrial processes Acid rain caused by industrial discharge of sulfur dioxide (by burning high -sulfur fossil fuels) Eutrophication by runoff containing detergents or fertilizers Underground storage tank leakage, leading to soil contamination, and hence aquifer contamination Inappropriate disposal of various solid wastes and, on a localized scale, littering Oil spills

Water Regulations § The Environmental Protection Agency regulates limits on the amount of certain contaminants in the water § The Food and Drug Administration limits contaminants in bottled water (don’t need to write)… § Drinking water, including bottled water, may reasonably be expected to contain at least small amounts of some contaminants. The presence of these contaminants does not necessarily indicate that the water poses a health risk. § You. Tube: Story of Bottled Water

Categories of Water Tests Biological § § Chemical Microorganisms such as fecal coliform bacteria (Escherichia coli), Cryptosporidium, and Giardia lamblia § § Dissolved Oxygen(DO) § Biochemical oxygen demand (BOD) Dissolved organics: Colored Dissolved Organic Matter (CDOM), Dissolved Organic Carbon (DOC) § Chemical oxygen demand (COD) § § Bethic Macroinvertebrates: § § Mollusca (Snails) § § Plecoptera (Stonefly) Ephemeropteroidea (Mayfly) Trichoptera (Caddisfly) Physical § § Temperature § § Color, Taste and Odor Nutrients, such as nitrogen and phosphorus § Total suspended solids (TSS) Dissolved metals and metalloids (lead, Mercury (element), arsenic, etc. ) § Nutrients, such as nitrogen and phosphorus § Dissolved salts and elements (sodium, chloride, potassium, calcium, manganese, magnesium) p. H § § Pesticides § § Pharmaceuticals Heavy Metals Hormone analogs Turbidity (clarity) Electrical Conductivity (salinity / TDS: Total Dissolved Solids)

Temperature § Thermal Pollution (increased water temperature) – from factories & power plants that use water for cooling – also due to removing trees that would have provided shade to streams and ponds. § Affects how much oxygen water can hold and determines types of organisms that can survive – Warm water holds less oxygen – decreases oxygen supply – killing fish juveniles which are vulnerable to small increases in temperature

Dissolved Oxygen Tools: Tests can be done with an electronic dissolved oxygen meter or using chemicals reactions in the Azide-Winkler titration method. Measurement: mg/L Causes: • More sunlight and warmer temperatures bring increased activity levels in plant and animal life. Oxygen enters the water by absorption directly from the atmosphere or by aquatic plant and algae photosynthesis. Oxygen is removed from the water by respiration and decomposition of organic matter. • Water gains oxygen as it flows over rocks and creates riffles. When water moves slower or becomes stagnant, it may cause DO concentration to decrease. • During rainy seasons, oxygen concentrations tend to be higher because the rain interacts with oxygen in the air as it falls. Effects: • More DO generally relates to “healthier” water (oxygen is necessary for most aquatic species to breath) - related to Biochemical Oxygen Demand (BOD): how fast biological organisms use up oxygen in a body of water. High B. O. D. means high pollution which can spread disease and become hazardous to humans

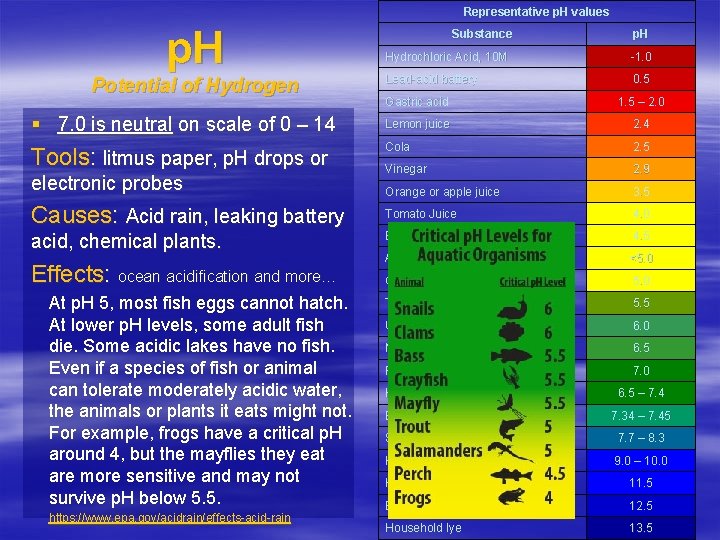
Representative p. H values p. H Potential of Hydrogen Substance p. H Hydrochloric Acid, 10 M -1. 0 Lead-acid battery 0. 5 Gastric acid 1. 5 – 2. 0 § 7. 0 is neutral on scale of 0 – 14 Lemon juice 2. 4 Tools: litmus paper, p. H drops or Cola 2. 5 Vinegar 2. 9 Orange or apple juice 3. 5 Tomato Juice 4. 0 Beer 4. 5 Acid Rain <5. 0 Coffee 5. 0 Tea or healthy skin 5. 5 Urine 6. 0 Milk 6. 5 Pure Water 7. 0 electronic probes Causes: Acid rain, leaking battery acid, chemical plants. Effects: ocean acidification and more… At p. H 5, most fish eggs cannot hatch. At lower p. H levels, some adult fish die. Some acidic lakes have no fish. Even if a species of fish or animal can tolerate moderately acidic water, the animals or plants it eats might not. For example, frogs have a critical p. H around 4, but the mayflies they eat are more sensitive and may not survive p. H below 5. 5. https: //www. epa. gov/acidrain/effects-acid-rain Healthy human saliva Blood 6. 5 – 7. 4 7. 34 – 7. 45 Seawater 7. 7 – 8. 3 Hand soap 9. 0 – 10. 0 Household ammonia 11. 5 Bleach 12. 5 Household lye 13. 5

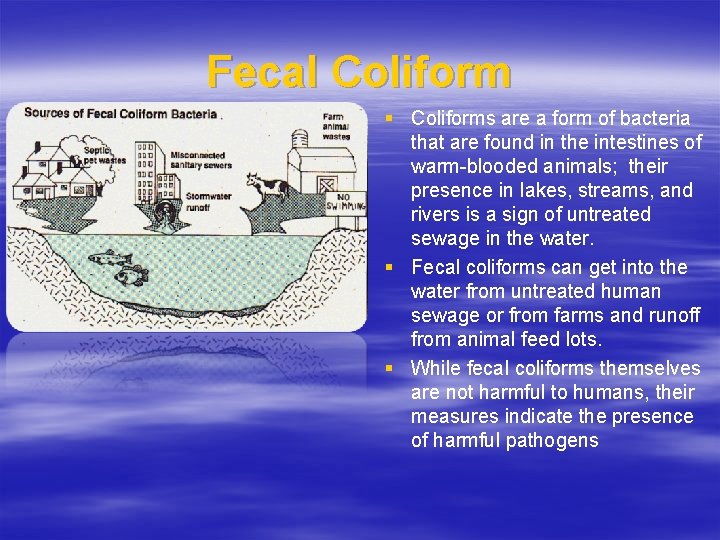
Fecal Coliform § Coliforms are a form of bacteria that are found in the intestines of warm-blooded animals; their presence in lakes, streams, and rivers is a sign of untreated sewage in the water. § Fecal coliforms can get into the water from untreated human sewage or from farms and runoff from animal feed lots. § While fecal coliforms themselves are not harmful to humans, their measures indicate the presence of harmful pathogens

Nutrients § Eutrophication, strictly speaking, means an increase in chemical nutrients (typically nitrogen and phosphorus) § Causes an increase in primary productivity – excessive plant growth and decay § Further impacts, including lack of oxygen and severe reductions in water quality and in fish and other animal populations.

Ammonia Tools for Measuring: § Ammonia test strips (p. H indicators) § Ammonia activator drops How to Measure: § Pour water into tube and place ammonia activator drops in and compare the color to the chart. Causes § Ammonia toxicity increases as temperature rises. § In aquariums, ammonia level rises due to different reasons: § Overfeeding, insufficient filtration, or dead fish decomposing in the tank. Effects of ammonia on people: § If inhaled can cause burning of nose, throat and respiratory failure. § If swallowed can result in damaged mouth, throat and stomach § Skin and eye irritation and burns Treatments: § Immediately wash with copious water § Drink milk or water to dilute ingested ammonia

Nitrogen: Ammonia NH 3 Nitrite NO 2 Nitrate NO 3 § § § Test: Nitrate Electrode Method Analyze: Read in millivolts converted to mg/L Causes: affects the electric potential of a solution in the probe. § Effects: Eutrophication in bodies of water. Can cause a restriction of oxygen transport in blood for humans.

Phosphorous / Phosphates § Measured with Colorimetric procedures and ascorbic acid treatment in units of mg/L § Phosphorous levels increase due to fertilizer or detergents § Higher levels promote algae growth which is detrimental to other organisms § Can be treated with calcium or aluminum and iron to lower phosphorous levels

Turbidity (Total Suspended Solids) § Measure of how clear or murky the water is based on amount of particulate matter (sediment, phytoplankton, nutrients, etc. ) is suspended in water. § Tools to measure: Electronic test kit or Secchi disk § Measured in Jackson Turbidity Units (JTU) or Nephelometric Turbidity Units (NTU) § Causes: sediments, algae, waste, urban runoff § Effects: absorb and block sunlight raising the biochemical oxygen demand (BOD) and reduces photosynthesis for aquatic plants.

Salinity Fresh water < 0. 05 % < 500 ppm Water salinity Brackish water Saline water 0. 05 - 3 % 500 - 30 000 ppm 3 - 5 % 30 000 - 50 000 ppm Brine > 5 % > 50 000 ppm § Test: Conductivity (Total Dissolved Solids) § § § Managing salinity involves striking a balance between the volume of water entering the groundwater system (recharge) and the volume of water leaving it (discharge). Causes: natural processes such as flooding and weathering of rocks deposit salt over thousands of years. When deep-rooted native plants are removed or replaced with shallow-rooted plants, they require more frequent watering which may draw salt from under the soil. Effects: may cause native vegetation to become unhealthy or die and lead to a decline in biodiversity through dominance of salt-resistant species. Also reduces agricultural crop production. Determines organisms that can live in that area. Oceans are about 35 ppt

Dissolved Metals • Many dissolved metals have been found in harmful concentrations in groundwater destined for potable drinking water due to both naturally occurring contamination as well as contamination introduced from industrial pollution. Examples- mercury, and lead, Cadium Where does mercury come from? Where does lead come from? • Mercury occurs in the environment • Lead is a natural element which is widely naturally, though it increases with erosion distributed in soils, rocks and in rivers and the sea. of rocks. But in nature lead usually exist combined with • Mercury can evaporate to form an other chemical elements in the form of lead odorless, colorless, vapor. Mercury was compounds. used for thermometers, but can be found • Lead poisoning is one of the most prevalent in fluorescent light bulbs. public health problems in many parts of the world. • This heavy metal is toxic even at low It was the first metal to be linked with failures in concentrations to organisms and humans reproduction. It can cross the placenta easily. It - the cause of several major epidemics of also affects the brain, causing hyperactivity and poisoning in humans resulting from the deficiency in the fine motor functions, thus, it ingestion of contaminated food, e. g. fish results in damage to the brain. • Ways to stop Mercury and lead pollution. Hazardous Wastes Management. Reducing products containing lead or mercury. Call the EPA to reduce the level of lead pollution, removed lead contents from gasoline, and check older buildings for lead.

Microorganisms § E. coli (fecal coliform bacteria) can generally cause several intestinal and extra-intestinal infections § Cryptosporidium is a protozoan pathogen and causes a diarrheal illness called cryptosporidiosis § Giardia lamblia is a flagellated protozoan parasite that colonizes and reproduces in the small intestine, causing giardiasis. Symptoms of include diarrhea, malaise, excessive gas, etc. § Some ways to get rid of these harmful substances is to boil water will kill all life within the water, carbon filters get rid of the bacteria though filtering, another way is to use a sand filter or evaporation method.

Pesticides bactericides, fungicides herbicides, insecticides (DDT) § Tools/Techniques- Regular tests are done to ensure public safety. For testing use a water testing kit or if you have a private well use private laboratories. § Measurement: The reporting limits for the HPAA method are 0. 2 micrograms per liter (ug/L), while reporting limits for the LCAA method are 0. 05 ug/L. § Cause : Pesticides not taken up by plants, adsorbed by soils or broken down by sunlight, soil organisms or chemical reactions may ultimately reach groundwater sources of drinking water. § Effects: Pesticides can cause cancer, organ damage in animals. In humans pesticides can cause damage to the nervous system, cause cancer, and birth defects. § Solutions: Use an activated carbon filter. Water is filtered through carbon granules that trap contaminants.

Pharmaceuticals v Pharmaceuticals are chemicals found in prescription, over-thecounter, and veterinary drugs. v Describe: The method used to test for pharmaceuticals combines solidphase extraction with high-performance liquid chromatography. v Analyze: Measured in nanograms per liter; usually present in much lower amounts than the acceptable medical dose v Causes: Pharmaceuticals are introduced to the water supply through the sewage of individuals who took these drugs, improper drug disposal, poorly controlled manufacturing facilities, and agricultural runoff. v Effects: Since the pharmaceuticals are present in such small amounts, there are usually no negative effects to human health. However, scientists are beginning to worry about the long-term consequences of these chemicals in drinking water. v Solutions: Natural processes (adsorption into sediment, solar photodegradation and biological degradation); proper household drug disposal; wastewater treatment