Water Quality Rice Creek Watershed Rice Creek Watershed










- Slides: 12

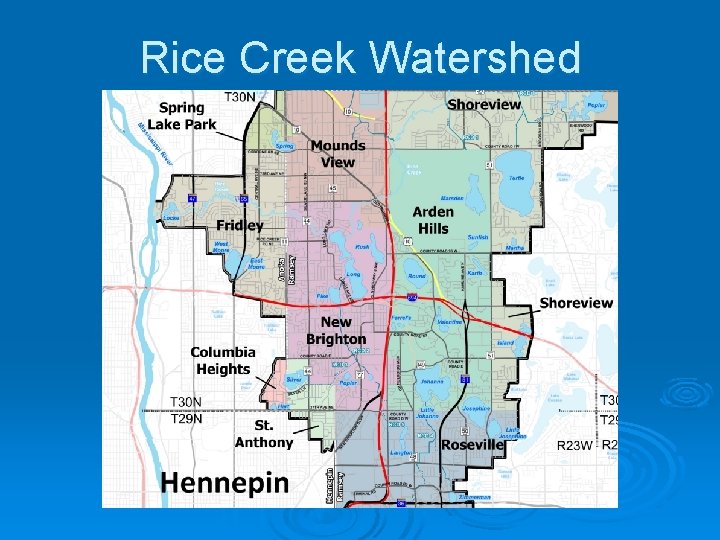
Water Quality Rice Creek Watershed

Rice Creek Watershed

Dissolved Oxygen Ø What is it? l measure of dissolved oxygen in the water Ø Why does it matter? l Clean, healthy water has plenty of DO. When water quality decreases, DO levels drop and it becomes impossible for many animals to survive. Time of year also matters. Ø How do DO levels in the water drop?

Biochemical Oxygen Demand Ø What is it? l measures the amount of oxygen consumed by microorganisms in the process of decomposing organic matter in water Ø Why does it matter? l A high BOD means less oxygen is available for other aquatic life, they become stressed, suffocate, and die. Ø How do BOD levels rise?

Coliform Bacteria Ø What is it? l Fecal coliform bacteria are naturally present in the human digestive tract but are rare or abesent in unpolluted water. Ø Why does it matter? l High levels of fecal coliform bacteria are a warning sign that water can make you sick. Ø How does fecal coliform get in the water?

p. H Ø What is it? l p. H is a measurement of the acidity or basic quality of water Ø Why does it matter? l At extremely high or low p. H levels (above 9. 6 or below 4. 5), the water becomes unsuitable for most organisms. Ø How do levels of p. H become too high or low?

Temperature Ø What is it? l Ø Temperature is a measure of how much heat is present in the water Why does it matter? l Temperature affects • Dissolved oxygen levels in water – cold holds more O 2 • Photosynthesis – high temps increases plant growth and decomposition, leading to decreased O 2 levels • Animal Survival – ideal temperature ranges for organisms • Sensitivity to toxic wastes and disease – waste generally increases water temps leading to lower O 2 levels. Ø How does water get warmer?

Nitrate What is it? Ø l Extra nitrogen in water leads to rapid plant growth Why does it matter? Ø l Can lead to eutrophication: 1. 2. 3. 4. 5. 6. 7. Ø Nitrogen enters the water Plants take up the nitrogen and grow Plants (algae) die and sink to the bottom Bacteria at the bottom decompose the dead plants, using up oxygen in the process Oxygen levels drop, killing fish or aquatic insects Nitrogen continues to enter the water The cycle continues How does too much nitrogen get in the water?

Phosphate What is it? Ø l Phosphorus is a nutrient found in all living things. Why does it matter? Ø l when too much phosphorus enters a river or lake, plants grow more…leads to eutrophication! l l l l Ø Phosphorus enters the water Plants take up the phosphorus and grow too much Plants (algae) die and sink to the bottom Bacteria at the bottom decompose the dead plants, using up oxygen in the process Oxygen levels drop, killing fish or aquatic insects Phosphorus continues to enter the water The cycle continues How does too much phosphorus get into the water?

Turbidity Ø What is it? l measures of how cloudy a water body is Ø Why does it matter? l When the water is turbid • floating particles absorb heat from the sun and cause the water temperature to rise • blocks sunlight • floating particles may clog fish gills Ø How do turbidity levels rise?

Conductivity Ø What is it? l Ø Why does it matter? l Ø Determines the total concentration of ions in a water sample. Gives general indication of the level of total dissolved solids in a lake or stream. These conductive ions come from dissolved salts and inorganic materials such as alkalis, chlorides, sulfides and carbonate compounds. How do inorganic pollutants get into water?

In your groups answer the following… How do DO levels in the water drop? How do BOD levels rise? How does fecal coliform get in the water? How do levels of p. H become too high or low? How does water get warmer? How does too much nitrogen get in the water? How does too much phosphorus get into the water? Ø How do turbidity levels rise? Ø How do inorganic pollutants get into water? Ø Ø Ø Ø