Water Quality Indicators Physical Indication of Water Pollution












- Slides: 17

Water Quality Indicators Physical Indication of Water Pollution

To a great extent, the success or failure of an aquatic ecosystem is determined by water quality

Water Quality Variables • Temperature • Dissolved oxygen • Total ammonia-nitrogen, NH 3, NO-2 • Alkalinity & Hardness • p. H • Turbidity • Salinity

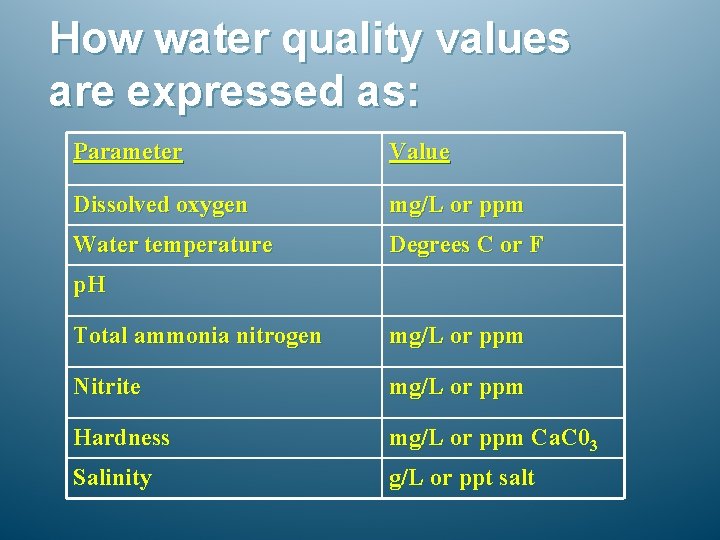
How water quality values are expressed as: Parameter Value Dissolved oxygen mg/L or ppm Water temperature Degrees C or F p. H Total ammonia nitrogen mg/L or ppm Nitrite mg/L or ppm Hardness mg/L or ppm Ca. C 03 Salinity g/L or ppt salt

Temperature • Normal temperature: 22 degrees C Sources: sunlight, thermal pollution Effects: amount of oxygen that can dissolve, photosynthetic rate, metabolic rates change, sensitivity to toxic wastes • Water temperature fluctuates seasonally, resulting in thermal stratification in deeper water.

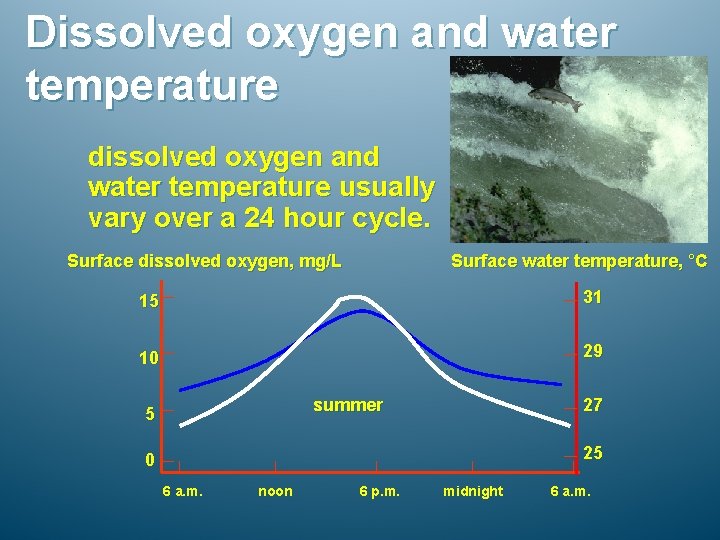
Dissolved oxygen and water temperature dissolved oxygen and water temperature usually vary over a 24 hour cycle. Surface dissolved oxygen, mg/L Surface water temperature, °C 15 31 10 29 summer 5 27 25 0 6 a. m. noon 6 p. m. midnight 6 a. m.

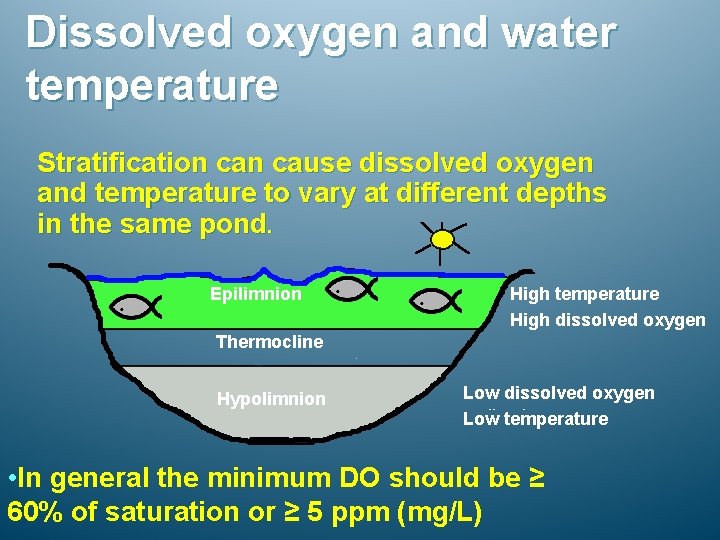
Dissolved oxygen and water temperature Stratification cause dissolved oxygen and temperature to vary at different depths in the same pond. Epilimnion High temperature High dissolved oxygen Thermocline Hypolimnion Low dissolved oxygen Low temperature • In general the minimum DO should be ≥ 60% of saturation or ≥ 5 ppm (mg/L)

Turbidity • Measures the amount of suspended particles in water • Too turbid, then what are the effects? – Temperature rises – Fish may have difficult finding a mate – Clogs fish gills

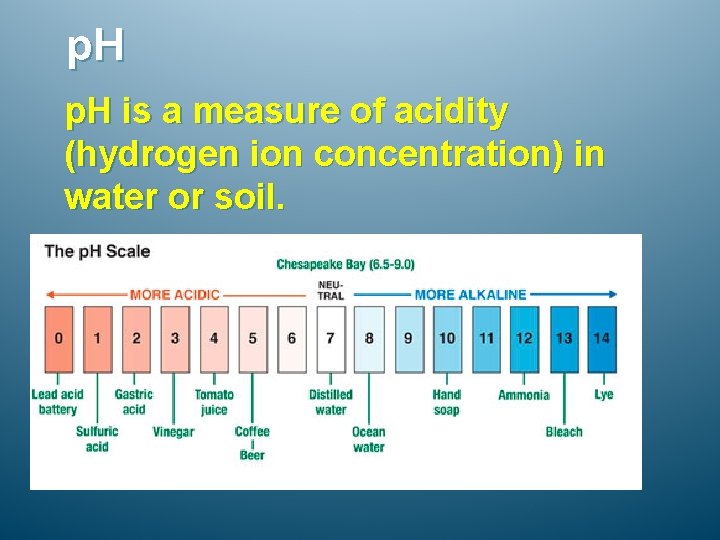
p. H is a measure of acidity (hydrogen ion concentration) in water or soil.

p. H Values of Water The acidity of water is gauged by its p. H , which is a measure of the concentration of the hydrogen ion (H + ) in the solution according to the relationship p. H = −log(H + ). The higher the concentration of H + in the water, the lower its p. H, and the greater its acidity. Acid waters have a p. H less than 7 (neutral p. H is 7), with the most acid waters at p. H 1 or less. Basic (alkaline) waters have a p. H greater than 7, with the most basic waters at p. H 14. The p. H range of freshwater is 6 to 8. The average p. H of sea (ocean water) is 8. 1.

Effects of low p. H Acid deposition has many harmful ecological effects when the p. H of most aquatic systems falls below 6 and especially below 5. Here are some effects of increased acidity on aquatic systems: • As the p. H approaches 5, non-desirable species of plankton and mosses may begin to invade, and populations of fish such as smallmouth bass disappear. • Below a p. H of 5, fish populations begin to disappear, the bottom is covered with un-decayed material, and mosses may dominate near shore areas. • Below a p. H of 4. 5, the water is essentially devoid of fish.

Effects of High p. H (basic) When the p. H of freshwater becomes highly alkaline (e. g. 9. 6), the effects on fish may include: • death, damage to outer surfaces like gills, eyes, and skin and an inability to dispose of metabolic wastes. • High p. H may also increase the toxicity of other substances. For example, the toxicity of ammonia is ten times more severe at a p. H of 8 than it is at p. H 7. It is directly toxic to aquatic life when it appears in alkaline conditions. Low concentrations of ammonia are generally permitted for discharge.

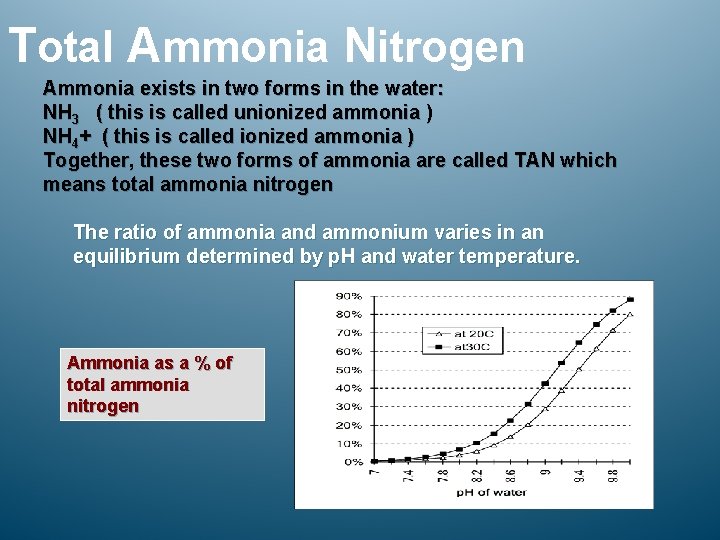
Total Ammonia Nitrogen Ammonia exists in two forms in the water: NH 3 ( this is called unionized ammonia ) NH 4+ ( this is called ionized ammonia ) Together, these two forms of ammonia are called TAN which means total ammonia nitrogen The ratio of ammonia and ammonium varies in an equilibrium determined by p. H and water temperature. Ammonia as a % of total ammonia nitrogen

Effects of TAN NH 3 is the principal form of toxic ammonia. It has been reported toxic to freshwater organisms at concentrations ranging from 0. 53 to 22. 8 mg/L. Toxic levels are both p. H and temperature dependent. Toxicity increases as p. H decreases and as temperature decreases. Plants are more tolerant of ammonia than animals, and invertebrates are more tolerant than fish. Hatching and growth rates of fishes may be affected. In the structural development, changes in tissues of gills, liver, and kidneys may also occur. Toxic concentrations of ammonia in humans may cause loss of equilibrium, convulsions, coma, and death.

Soft and Hard Water hardness and softness has nothing to do with its touch and feel. It is more about chemical compounds dissolved in it. They are both safe for human consumption. Pure water (like clean rainwater) is soft water. It only becomes hard when it comes into contact with rock layers made up of compounds such as calcium or magnesium, and dissolves in it.

Hard Water vs Soft Water When it boils down, the major difference between hard and soft water can best be seen while doing household chores. Hard water is to blame for dingy looking clothes, dishes with spots and residue, and bathtubs with lots of film and soap scum. Even hair washed in hard water may feel sticky and look dull. Hard water can take a toll on household appliances as well and use up more energy. The elements of hard water are to blame for all of these negative factors, as soap is less effective due to its reaction to the magnesium and calcium. The lather is not as rich and bubbly.

Salinity Fresh water is less than 2 g/L Brackish water is 2 g/L to 34 g/L Na. Cl Sea water is more than 34 g/L
 Water and water and water water
Water and water and water water Ahrq quality indicators
Ahrq quality indicators Prevention quality indicators
Prevention quality indicators Ahrq quality indicators toolkit
Ahrq quality indicators toolkit Environmental indicators of physical child abuse
Environmental indicators of physical child abuse Quality control and quality assurance
Quality control and quality assurance Pmp quality vs grade
Pmp quality vs grade Pmbok quality management
Pmbok quality management Define quality assurance in nursing
Define quality assurance in nursing Quality improvement vs quality assurance
Quality improvement vs quality assurance Qa basic concepts
Qa basic concepts David a garvin 8 dimensions of quality
David a garvin 8 dimensions of quality Crosby's fourteen steps to quality improvement
Crosby's fourteen steps to quality improvement Old quality vs new quality
Old quality vs new quality Source of contamination
Source of contamination Section 3 water pollution
Section 3 water pollution Water pollution through the years
Water pollution through the years Three source of water
Three source of water