Water Quality and Testing Water Quality Water quality















- Slides: 18

Water Quality and Testing

Water Quality • Water quality is the physical, chemical and biological characteristics of water • The vast majority of surface water on the planet is neither potable (fit for drinking) nor toxic • Approximately 25% of the world’s population has no access to potable water

Water Quality • No simple property can tell whether water is polluted or not • Industrial pollution is a major cause of water pollution – Also runoff from agricultural areas, urban storm water runoff and discharge of untreated sewage (especially in developing countries).

Water Contamination • Contaminants that may be in untreated water include: – – – microorganisms (viruses and bacteria) inorganic contaminants (salts and metals) pesticides and herbicides sediments organic chemical contaminants from industrial processes and petroleum use – radioactive contaminants. • Water quality depends on the local geology and ecosystem, as well as human uses (sewage dispersion, industrial pollution, use of water bodies as a heat sink)

Water Regulations • The Environmental Protection Agency (EPA) regulates limits on the amount of certain contaminants in the water • The Food and Drug Administration (FDA) limits contaminants in bottled water • Drinking water, including bottled water, may reasonably be expected to contain at least small amounts of some contaminants. The presence of these contaminants does not necessarily indicate that the water poses a health risk.

Water Tests • • • • • Electrical conductivity|Conductivity (also see salinity) Dissolved Oxygen(DO) p. H Color of water Taste and odor (geosmin, 2 -methylisoborneol (MIB), etc) Turbidity Total suspended solids (TSS) Dissolved metals and salts (sodium, chloride, potassium, calcium, manganese, magnesium) Chemical oxygen demand (COD) Biochemical oxygen demand (BOD) Microorganisms such as fecal coliform bacteria (Escherichia coli), Cryptosporidium, and Giardia lamblia Nutrients, such as nitrogen and phosphorus Dissolved metals and metalloids (lead, Mercury (element), arsenic, etc. ) Dissolved organics: Colored Dissolved Organic Matter (CDOM), Dissolved Organic Carbon (DOC) Temperature Pesticides Heavy Metals Pharmaceuticals Hormone analogs

Temperature • Determine types of organisms that can live in water • Affects how much oxygen water can hold – Warm water holds less oxygen • Thermal Pollution (increased water temperature) – decreasing oxygen supply – killing fish juveniles which are vulnerable to small increases in temperature – affecting ecosystem composition.

Dissolved Oxygen • Measure of the amount of oxygen dissolved in water • Measurement for outdoor bodies of water – More DO generally relates to “healthier” water (oxygen necessary for most aquatic species to breath) • Related to Biochemical Oxygen Demand (BOD) – how fast biological organisms use up oxygen in a body of water

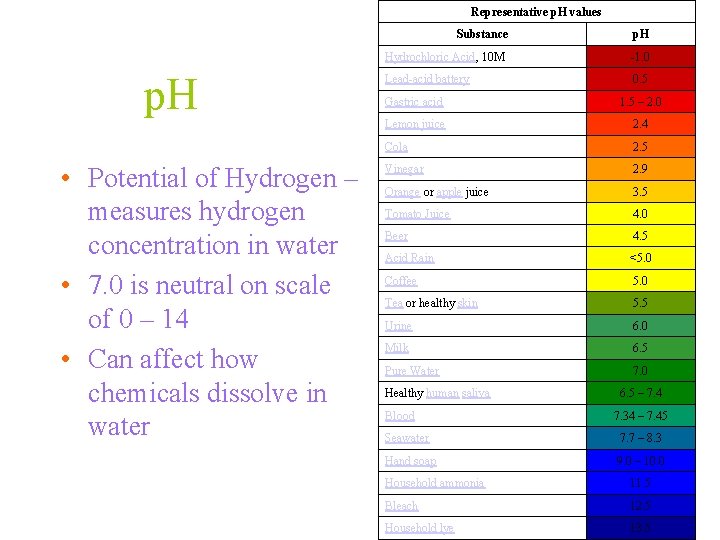
Representative p. H values Substance p. H • Potential of Hydrogen – measures hydrogen concentration in water • 7. 0 is neutral on scale of 0 – 14 • Can affect how chemicals dissolve in water p. H Hydrochloric Acid, 10 M -1. 0 Lead-acid battery 0. 5 Gastric acid 1. 5 – 2. 0 Lemon juice 2. 4 Cola 2. 5 Vinegar 2. 9 Orange or apple juice 3. 5 Tomato Juice 4. 0 Beer 4. 5 Acid Rain <5. 0 Coffee 5. 0 Tea or healthy skin 5. 5 Urine 6. 0 Milk 6. 5 Pure Water 7. 0 Healthy human saliva Blood 6. 5 – 7. 4 7. 34 – 7. 45 Seawater 7. 7 – 8. 3 Hand soap 9. 0 – 10. 0 Household ammonia 11. 5 Bleach 12. 5 Household lye 13. 5

Nutrients • Eutrophication, strictly speaking, means an increase in chemical nutrients (typically nitrogen and phosphorus) • Resultant increase in primary productivity – excessive plant growth and decay • Further impacts, including lack of oxygen and severe reductions in water quality and in fish and other animal populations.

Turbidity / Total Suspended Solids • Amount of particulate matter in water • Related to sediment, phytoplankton and nutrients in water • Turbidity reflects matter visible to naked eye (measured with secchi disk) • TSS collects dissolved material (filtered) measured as weight

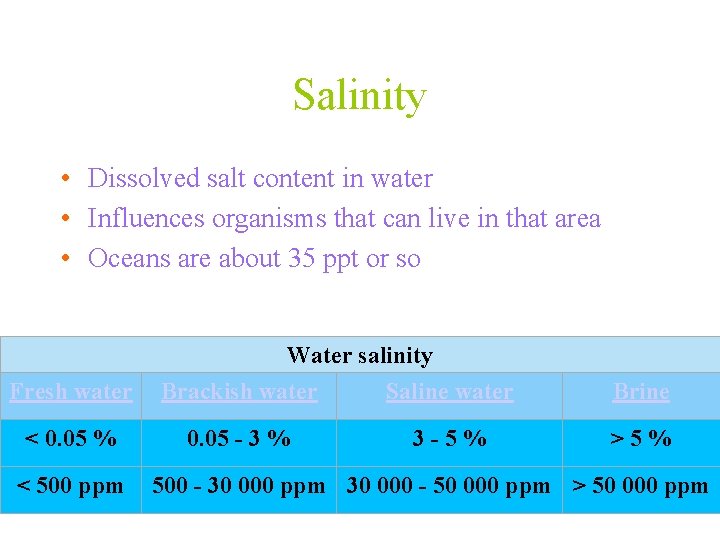
Salinity • Dissolved salt content in water • Influences organisms that can live in that area • Oceans are about 35 ppt or so Fresh water < 0. 05 % < 500 ppm Water salinity Brackish water Saline water 0. 05 - 3 % 3 - 5 % Brine > 5 % 500 - 30 000 ppm 30 000 - 50 000 ppm > 50 000 ppm

Heavy Metals • Living organisms require trace amounts of some heavy metals, including iron, cobalt, copper, manganese, molybdenum, vanadium, strontium, and zinc – excessive levels can be detrimental to the organism. • Other heavy metals such as mercury, lead and cadmium are toxic metals – they have no known vital or beneficial effect on organisms – their accumulation over time in the bodies of mammals can cause serious illness

Microorganisms • E. coli (fecal coliform bacteria) can generally cause several intestinal and extra-intestinal infections • Cryptosporidium is a protozoan pathogen and causes a diarrheal illness called cryptosporidiosis • Giardia lamblia is a flagellated protozoan parasite that colonises and reproduces in the small intestine, causing giardiasis. Symptoms of include diarrhea, malaise, excessive gas, etc

Other Stuff • Pesticides - bactericides, fungicides herbicides, insecticides (DDT) • Radioactive Materials • A lot of this information pertains to soil testing as well.

Sources of Water Pollution • • • Industrial discharge of chemical wastes and byproducts Discharge of poorly-treated or untreated sewage Surface runoff containing pesticides or fertilizers Slash and burn farming practice, which is often an element within shifting cultivation agricultural systems Surface runoff containing spilled petroleum products Surface runoff from construction sites, farms, or paved and other impervious surfaces Discharge of contaminated and/or heated water used for industrial processes Acid rain caused by industrial discharge of sulfur dioxide (by burning high-sulfur fossil fuels) Eutrophication by runoff containing detergents or fertilizers Underground storage tank leakage, leading to soil contamination, and hence aquifer contamination Inappropriate disposal of various solid wastes and, on a localized scale, littering Oil spills

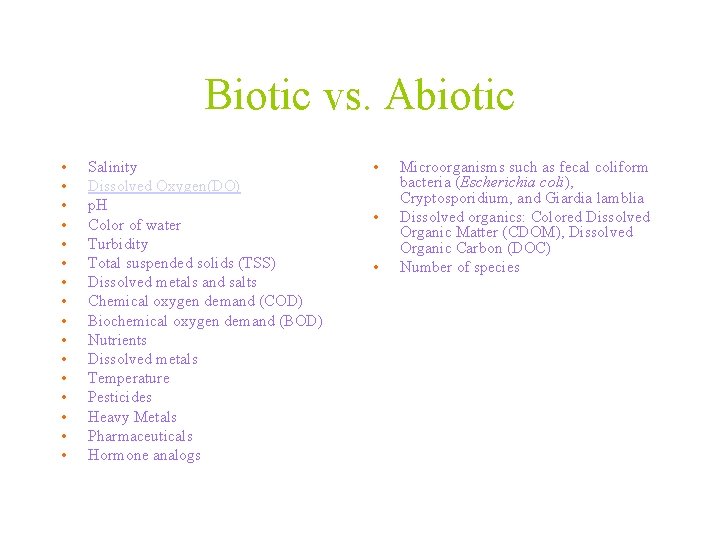
Biotic vs. Abiotic • Abiotic components are non-living chemical and physical factors in the environment. – The six major aboiotic factors are water, sunlight, oxygen, temperature, soil and climate. • Biotic means relating to, produced by, or caused by living organisms.

Biotic vs. Abiotic • • • • Salinity Dissolved Oxygen(DO) p. H Color of water Turbidity Total suspended solids (TSS) Dissolved metals and salts Chemical oxygen demand (COD) Biochemical oxygen demand (BOD) Nutrients Dissolved metals Temperature Pesticides Heavy Metals Pharmaceuticals Hormone analogs • • • Microorganisms such as fecal coliform bacteria (Escherichia coli), Cryptosporidium, and Giardia lamblia Dissolved organics: Colored Dissolved Organic Matter (CDOM), Dissolved Organic Carbon (DOC) Number of species