Water Purification Differences in density and miscibility Water

Water Purification: Differences in density and miscibility • Water is polar. – Recall polar = having poles, + and -. • Non-polar substances do not dissolve in polar ones--Like dissolves like. • Non-polar and polar substances are immiscible--they do not mix. • If you let a mixture of oil and water settle, they will separate. • Which layer is on top? – (hint--think of salad dressing)

Water Purification: Filtration • Filtrate = liquid collected after filtration • Sand filter = Traps and removes larger solid impurities • Charcoal = Adsorbs foreign substances in water. • Adsorb = To attract and hold impurities • Removes substances that cause bad taste, odor or cloudy appearance • Used in fish aquariums
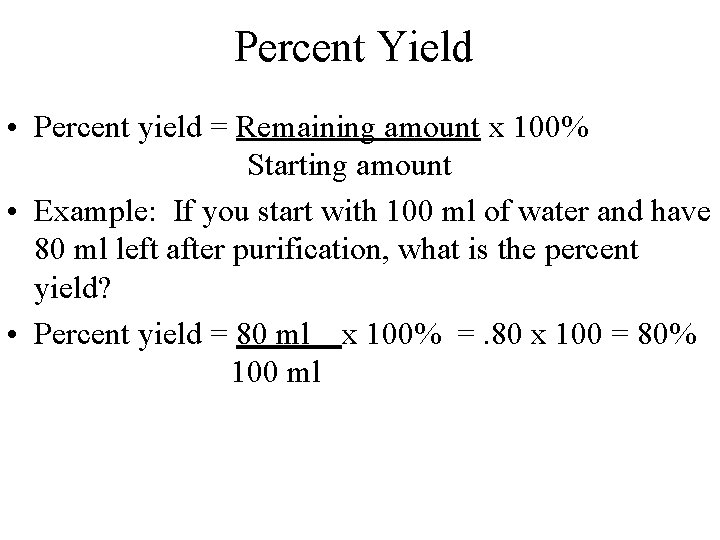
Percent Yield • Percent yield = Remaining amount x 100% Starting amount • Example: If you start with 100 ml of water and have 80 ml left after purification, what is the percent yield? • Percent yield = 80 ml x 100% =. 80 x 100 = 80% 100 ml
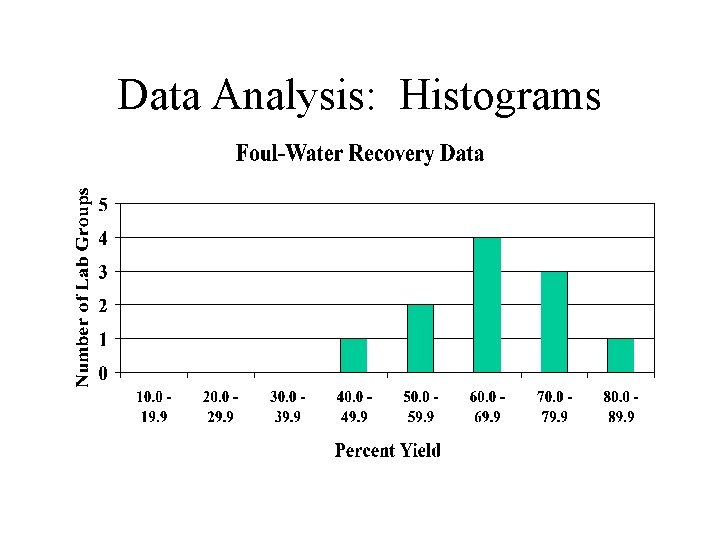
Data Analysis: Histograms

Purification: Distillation • Distillation is used to separate mixtures based on differences in boiling points. • Liquid with lower boiling point evaporates first. • The evaporated liquid condenses as it passes through the cool condensation tube. • The condensed liquid drains into the clean container below.

Properties of solutions and mixtures • A solution that contains ions will conduct electricity. – Recall that an ion is an element or compound which has gained or lost electron(s). • Electrical conductivity can be measured with a conductivity meter. – Any circuit that will indicate passing of electricity can serve as a qualitativie conductivity meter. • The Tyndall effect is the incidence of light passing through a mixture due to particles being large enough to reflect light.

Hard Water Chemistry in the Community, by the ACS, W. H. Freeman and Co. , 2002 • Excess of Ca 2+ , Mg 2+, and/or Fe 3+ dissolved in water results in “hard” water. • In nature, as groundwater flows over limestone, chalk and other minerals containing calcium, magnesium, and iron, these ions may be dissolved • Man-made pollutants may also cause hard water • Hard water does not form a soapy lather easily, therefore interfering with the desired cleaning action of soap. • In hard water, soap reacts with hard water ions to form insoluble compounds: Soap scum or bathtub ring
- Slides: 7