Water Properties Density The amount of atoms or



























































![Practice Surface Tension • . . My Documentsgrays creek20112012Hydrosphere videosSurface tension [www. keepvid. com]. Practice Surface Tension • . . My Documentsgrays creek20112012Hydrosphere videosSurface tension [www. keepvid. com].](https://slidetodoc.com/presentation_image_h/03430242dbb2e39685d48bee94bd1981/image-61.jpg)













- Slides: 76

Water Properties

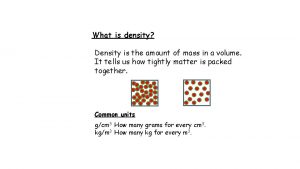
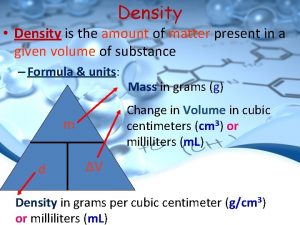
Density • The amount of atoms, or stuff, packed into the same space.



Which is more dense? • Cold or Hot Water – Cold water-things that are cold become more dense as they shrink • The only exception is ice, cold water becomes more dense until it freezes. It then expands!

Quickly watch water temperature and density video • . . My Documentsgrays creek20112012Hydrosphere videosdensity tank, cold salt warm fresh. mp 4

Temperature affect on ocean water • Think back to our dissolved oxygen lab • What happened as the temperature rose with relation to the amount of oxygen in the water? • The same thing happens with salt in water • Warmer the water-less salty-less dense • Colder the water-more salt-more dense

Quickly watch density of salt water video • . . My Documentsgrays creek20112012Hydrosphere videosBill Nye The Science Guy on salinity levels. mp 4

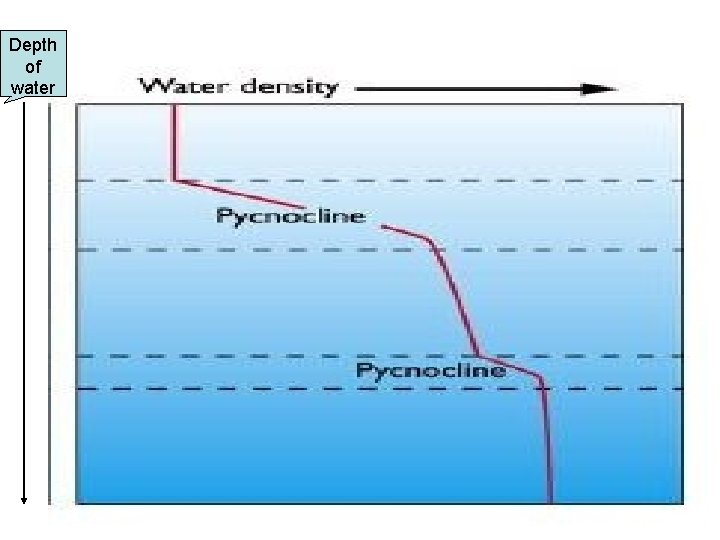
Density of Salt Water • What happens to the density of water the deeper you go? • It increases in density • Why? • The pressure from the water above is pushing things tighter and tighter!

Watch NASA Video on Temperature vs. Salinity • . . My Documentsgrays creek20112012Hydrosphere videosNASA Earth Science Week - temp vs. salintiy. mp 4

Depth of water

What variables might change the salinity of the oceans? • What if it rained near a very salty part of the ocean? • Precipitation would add more water and have less salt. • What if lots of water evaporated near a salty part of a ocean? • Evaporation will have less water, more salt.


Which is more dense? • Salt water or freshwater? – Salt Water of course • Why? – There is more “stuff” in the water.

Practice • I will be calling groups back to practice the density of salt water, cold water, and warm water. • While you are at your seats you WILL work on the following • 39 -50 in workbook • Finish your vocabulary assignment for this week.

Buoyancy • Buoyancy-the upward force caused by the difference in densities, or more simply-the ability of things to float • How is Buoyancy related to density? • Things float because of density-if you are less dense than something you will float




Watch Buoyant force video!

Practice Buoyancy

Title: How does density create buoyancy? • Date: Today’s Date • Directions: Answer all questions and perform all activities

• 1. Fill your 2 liter bottle that has been cut in half or your beaker about ¾ full of water. • 2. Place your boat that is on your desk on top of the water. • 3. What happens? • 4. Why does it float? • 5. How does the boat floating represent buoyancy?

• 6. Remove your boat • 7. What will happen when you throw a penny into the water? Why? • 8. How could we get our penny to float using buoyancy of another object? • 9. Place your boat into the water. Place pennies into your boat until the density becomes too great for buoyancy to keep them afloat.

• 10. How many pennies did it take counteract the upward force of buoyancy? • Review Questions: – 1. What is buoyancy? – 2. Why is buoyancy important for boats?

Show to Stick or Not To Stick Video

Cohesion • Ability of like substances to stick together • Water binds to other water molecules to create the cohesion of water.

Cohesion • THIS IS WHY WATER DROPS LOOK A LITTLE FUNNY • What happens when you do a belly flop on the water? • You are breaking the cohesive bond between water!



Practice

Title: Cohesion of water molecules • 1. Place 1 drop of water onto a penny. • 2. Draw a picture from the side of what you see. • 3. Why does the water drop have a dome shape to it? • 4. Keep placing drops on water onto your penny until the drops overflow, be sure to count

• 5. How many drops did it take? • 6. Draw a picture of what your penny looked like before you dropped it. • 7. What might have caused your dome of to break? • 8. Repeat the process of making a large dome of water. Then take 1 drop of soapy water and observe what happens.

• Review: – 1. What is cohesion? – 2. Why do you think the soap broke the cohesion of the water? – 3. Why is cohesion important?

Adhesion • Water molecules have the ability to stick to different substances, sometimes they are absorbed, sometimes they are not.







Show to stick or not to stick part of video on adhesion

Adhesion Observations

Title: Adhesion of water to wood • 1. Using your water bottle on your desk, place your ruler at an angle above the empty cut 2 liter bottle of water. • 2. Do your best to squirt the top of the ruler and make the water end up in the empty two liter bottle.

• Review: – 1. What is adhesion? – 2. How did you demonstrate adhesion? – 3. Why do you think adhesion is important to the environment?

Capillary Action • The ability of water to “climb”, or defy gravity. • This is how plants get their water and how paper towels absorb water.

• The adhesion, ability of water to stick to other substances, allows water to “climb” • The attraction between the paper towel and water is adhesive or its the stickiness of water.





Practice Capillary Action • 1. Holding one end of your paper towel, dip the other end into the colored water and watch it “climb”.

• Review – 1. What is capillary action? – 2. What causes capillary action? – 3. Why is capillary action so important in nature?

Surface Tension • The cohesive and adhesive properties of water create surface tension. • Surface Tension-the strength between water molecules on the surface of water.






Watch surface tension video • . . My Documentsgrays creek20112012Hydrosphere videosSurface tension [www. keepvid. com]. mp 4
![Practice Surface Tension My Documentsgrays creek20112012Hydrosphere videosSurface tension www keepvid com Practice Surface Tension • . . My Documentsgrays creek20112012Hydrosphere videosSurface tension [www. keepvid. com].](https://slidetodoc.com/presentation_image_h/03430242dbb2e39685d48bee94bd1981/image-61.jpg)
Practice Surface Tension • . . My Documentsgrays creek20112012Hydrosphere videosSurface tension [www. keepvid. com]. mp 4

Title: Surface Tension of Water • • 1. Fill your plastic cup up almost to the top 2. Toss one paper clip into the water. 3. What happened and why? 4. Attempt to place 1 paper clip so that it stays on top of the surface of the water and does not sink. • 5. Record your observations about what you notice about the water once you get one to float.

Specific Heat • Water has a very high specific heat • Specific Heat-the amount of heat needed to heat up a substance • Water takes a long time to boil and will stay warm for a long time.


Specific Heat • The beach in summer time • Sand- cold in morning-Hot in afternoon • Water-stays about the same temperature all day and all night

Specific Heat • The ocean water will not warm up until late June. It will stay warm until late Sept. • Why does it take so long to warm and yet stay warm for a long time?

Watch Specific Heat video

Why did the ice cube melt faster on the mystery object? • The mystery element has a lower specific heat therefore it will absorb heat faster.

Practice Specific Heat • 1 drop of alcohol on desk. • 1 drop of water – Swirl both around and make an observation – 1. What was the difference in the liquids? – 2. What can explain this?

Universal Solvent • What does it mean when we say that water is the universal solvent? • water can dissolve more things than any other substance • Why do things dissolve in substance? • They are soluble or they have the same polarity. • Like dissolves like. The closer the polarity, the charge, the more likely they will dissolve.

Watch solute and solvent video

Polarity • Attraction between water, the universal solvent, and other substances will cause things to dissolve in them. • Polar=Dissolve • Non-Polar=will not dissolve

Watch Soluble vs. Insoluble Video

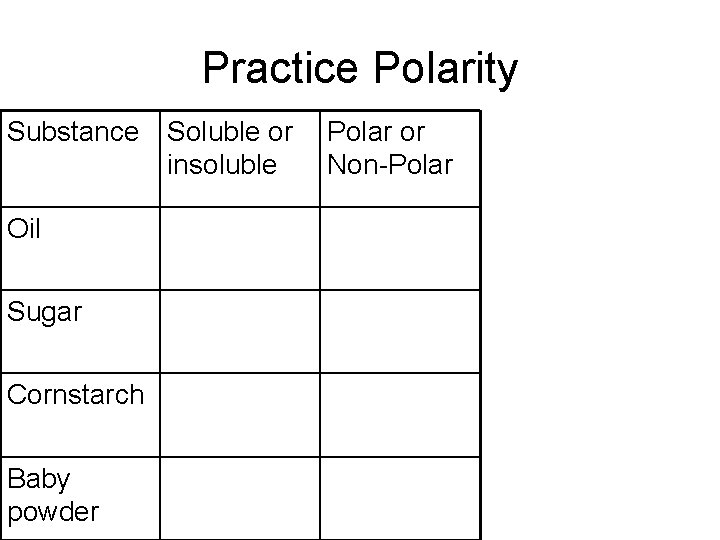
Practice Polarity Substance Soluble or insoluble Oil Sugar Cornstarch Baby powder Polar or Non-Polar

Review of Polarity • 1. What is the relationship between polarity and solubility?

Tides • What are tides? • Tides are changes in the ocean depths at the beach. • What causes tides? • The gravitational pull of the moon as it rotates around the Earth. • The oceans, as a result of cohesion move as one.
 Water and water and water water
Water and water and water water Periodic table of elements regents
Periodic table of elements regents Linear density of fcc 111
Linear density of fcc 111 Linear density of atoms
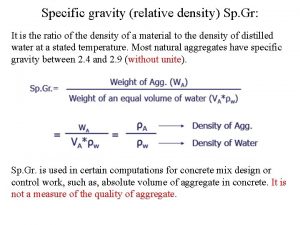
Linear density of atoms What is the difference between density and relative density
What is the difference between density and relative density Physiological density
Physiological density Nda full dac
Nda full dac Physiological density of egypt
Physiological density of egypt Properties of atoms and the periodic table
Properties of atoms and the periodic table Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Chemical properties of atoms
Chemical properties of atoms Brain pop density
Brain pop density Water density sensors
Water density sensors Which weighs more
Which weighs more Fresh water density
Fresh water density Displacement method volume
Displacement method volume Fwa in ship
Fwa in ship Dmv triangle science
Dmv triangle science Snc1d chemistry
Snc1d chemistry Intensive vs extensive
Intensive vs extensive Physical and chemical properties
Physical and chemical properties Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng chữ đua
Môn thể thao bắt đầu bằng chữ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng bé xinh thế chỉ nói điều hay thôi
Cái miệng bé xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Số nguyên tố là
Số nguyên tố là Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Concept map on water
Concept map on water Properties of water ap bio
Properties of water ap bio Characteristics of pure water
Characteristics of pure water Properties of water clipart
Properties of water clipart Water properties lab
Water properties lab Ocean water properties
Ocean water properties Properties of water in matter
Properties of water in matter Bill nye surface tension
Bill nye surface tension Honors biology properties of water lab
Honors biology properties of water lab The extraordinary properties of water
The extraordinary properties of water The extraordinary properties of water
The extraordinary properties of water Properties of water foldable
Properties of water foldable