Water Potential Osmosis Plant cells Plants water potential






- Slides: 13

Water Potential Osmosis & Plant cells

Plants & water potential • Plants can use the potential energy in water to perform work. • Tomato plant regains turgor pressure – cell pushes against wall due to uptake of water

Plants & water potential • The combined effects of 1. ) solute concentration 2. ) physical pressure (cell wall) can be measured as Water Potential • is measured in kilopascals (k. Pa) or bars • 1 Mpa = 10 atmospheres of pressure or 10 bars

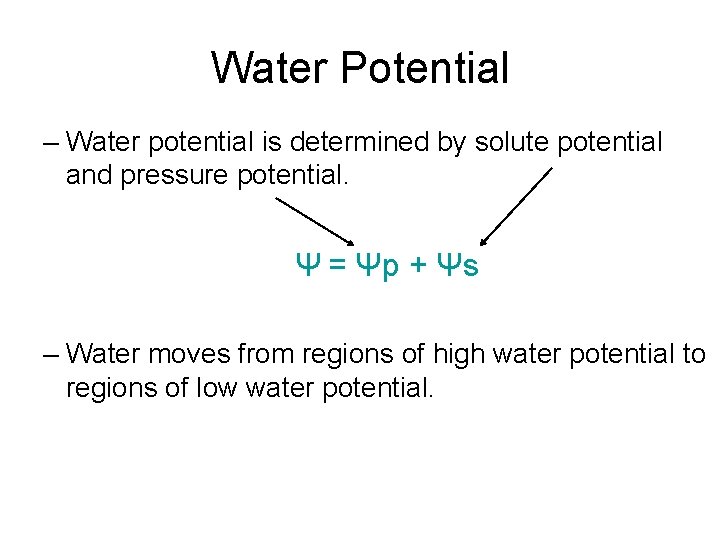
Water Potential – Water potential is determined by solute potential and pressure potential. Ψ = Ψp + Ψs – Water moves from regions of high water potential to regions of low water potential.

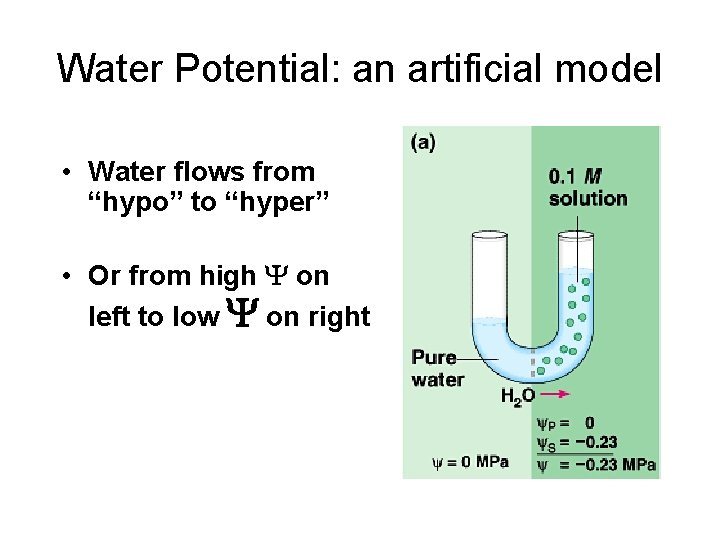
Water Potential: an artificial model • Water flows from “hypo” to “hyper” • Or from high on left to low on right

Pressure Potential – the sum of all pressure on water. • Turgor pressure – force caused by cell membrane pushing against cell wall. • Wall pressure – an equal and opposite force exerted by cell wall. • Other pressures – tension, cohesion, atmospheric, root, etc. • When working problems, use zero for pressure potential in animal cells & open beakers.

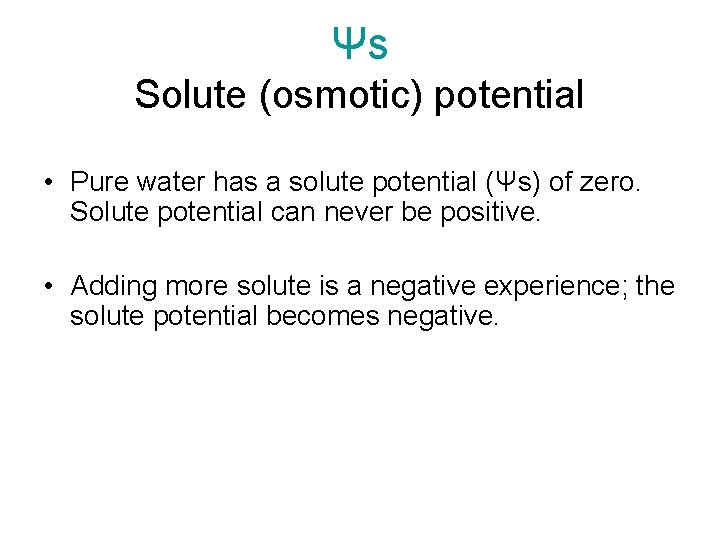
Ψs Solute (osmotic) potential • Pure water has a solute potential (Ψs) of zero. Solute potential can never be positive. • Adding more solute is a negative experience; the solute potential becomes negative.

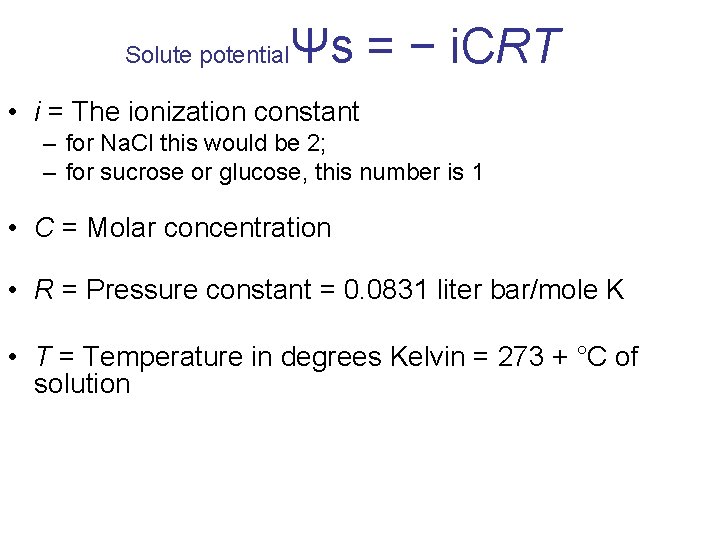
Ψs Solute (osmotic) potential Once you know the solute concentration, you can calculate solute potential using the following formula: • Solute potential (ΨS ) = –i. CRT

Solute potential Ψs = − i. CRT • i = The ionization constant – for Na. Cl this would be 2; – for sucrose or glucose, this number is 1 • C = Molar concentration • R = Pressure constant = 0. 0831 liter bar/mole K • T = Temperature in degrees Kelvin = 273 + °C of solution

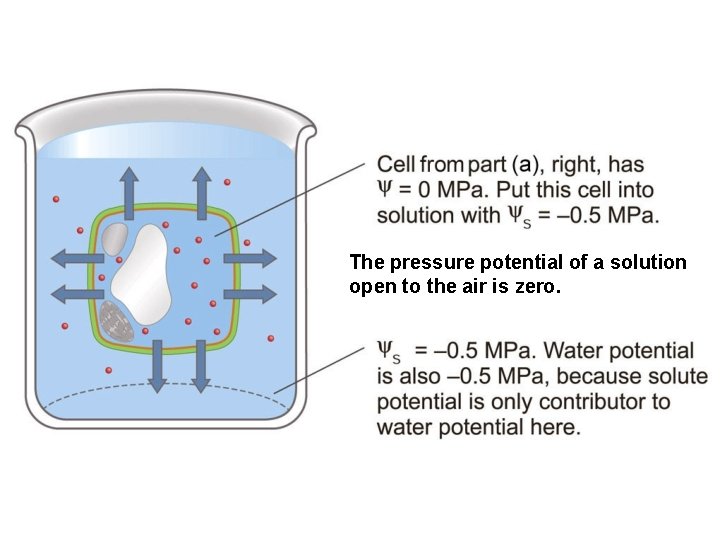
Water potential The pressure potential of a solution open to the air is zero.

Practice Problem • What is the water potential of a cell with a solute potential of -0. 67 k. Pa and a pressure potential of 0. 43 k. Pa?

Practice Problem • The molar concentration of a sugar solution in an open beaker has been determined to be 0. 5 M. Calculate the solute potential at 24°C. Round your answer to the nearest hundredth.
