Water Potential AP Biology Day 13 Water Potential
Water Potential AP Biology
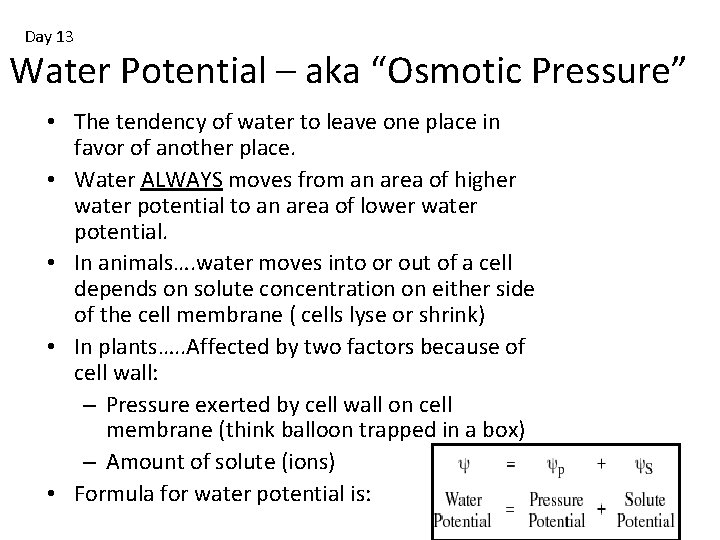
Day 13 Water Potential – aka “Osmotic Pressure” • The tendency of water to leave one place in favor of another place. • Water ALWAYS moves from an area of higher water potential to an area of lower water potential. • In animals…. water moves into or out of a cell depends on solute concentration on either side of the cell membrane ( cells lyse or shrink) • In plants…. . Affected by two factors because of cell wall: – Pressure exerted by cell wall on cell membrane (think balloon trapped in a box) – Amount of solute (ions) • Formula for water potential is:

• In plants, pressure exerted by the rigid cell wall that limits further water uptake (also known as TURGOR PRESSURE) • Pure water has solute potential of ZERO. As solute is added, the value for solute potential becomes more negative. This causes the water potential to decrease also. – As solute is added, the water potential of a solution drops, and water will tend to move into the solution. • In the laboratory, we use “bars” as the unit of measure for water potential. – 1 bar = approximately 1 atm
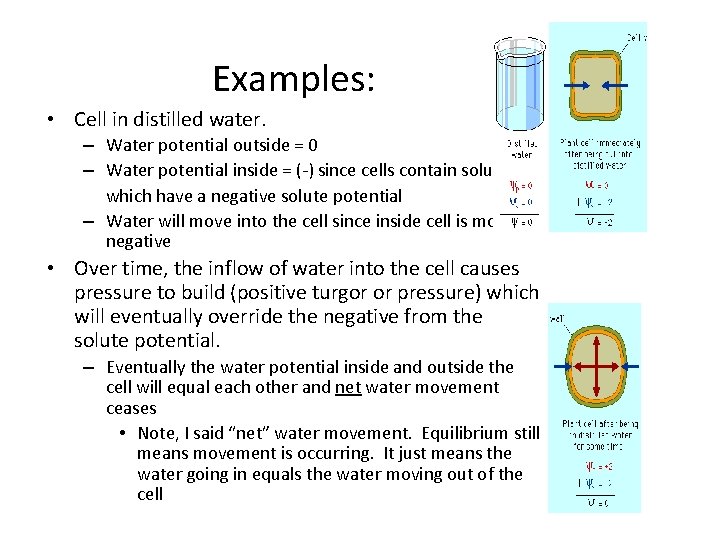
Examples: • Cell in distilled water. – Water potential outside = 0 – Water potential inside = (-) since cells contain solutes which have a negative solute potential – Water will move into the cell since inside cell is more negative • Over time, the inflow of water into the cell causes pressure to build (positive turgor or pressure) which will eventually override the negative from the solute potential. – Eventually the water potential inside and outside the cell will equal each other and net water movement ceases • Note, I said “net” water movement. Equilibrium still means movement is occurring. It just means the water going in equals the water moving out of the cell
- Slides: 4