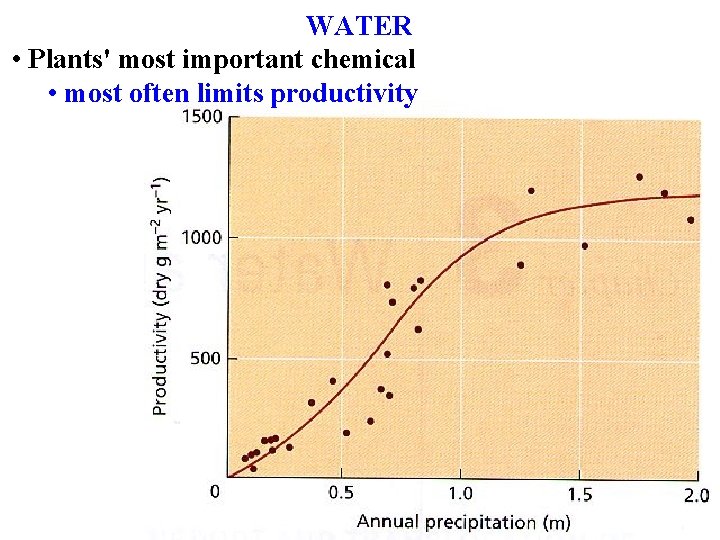
WATER Plants most important chemical most often limits




![p. H [H+] = acidity of a solution p. H = convenient way to p. H [H+] = acidity of a solution p. H = convenient way to](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-27.jpg)
![Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-29.jpg)
![Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-30.jpg)
![Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-31.jpg)
![Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-32.jpg)
![Water movement Diffusion: movement of single molecules down ∆[] due to random motion until Water movement Diffusion: movement of single molecules down ∆[] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-33.jpg)
![Water movement Diffusion: movement of single molecules down [] due to random motion until Water movement Diffusion: movement of single molecules down [] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-34.jpg)
![Water movement Diffusion: movement of single molecules down ∆[] due to random motion until Water movement Diffusion: movement of single molecules down ∆[] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-36.jpg)

![Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-45.jpg)
![Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-46.jpg)
![Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-47.jpg)
![Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-48.jpg)



- Slides: 61

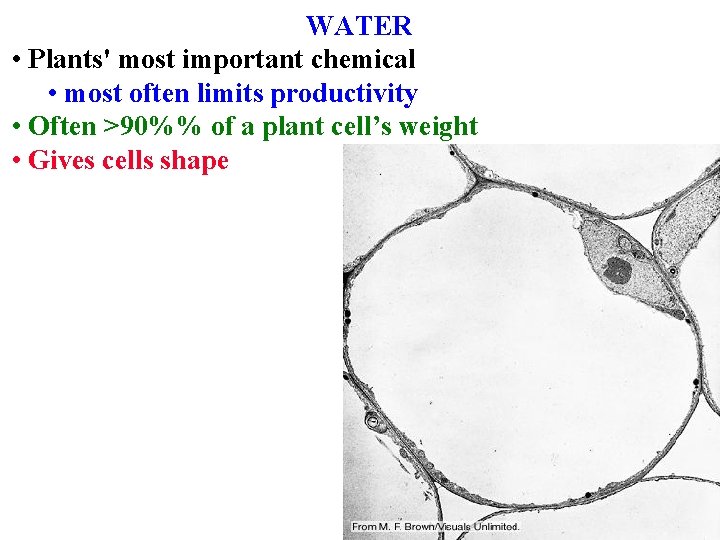
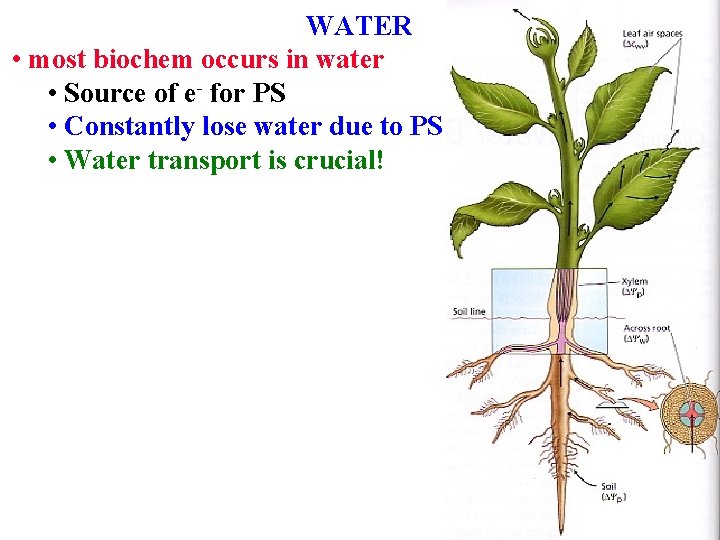
WATER • Plants' most important chemical • most often limits productivity

WATER • Plants' most important chemical • most often limits productivity • Often >90%% of a plant cell’s weight

WATER • Plants' most important chemical • most often limits productivity • Often >90%% of a plant cell’s weight • Gives cells shape

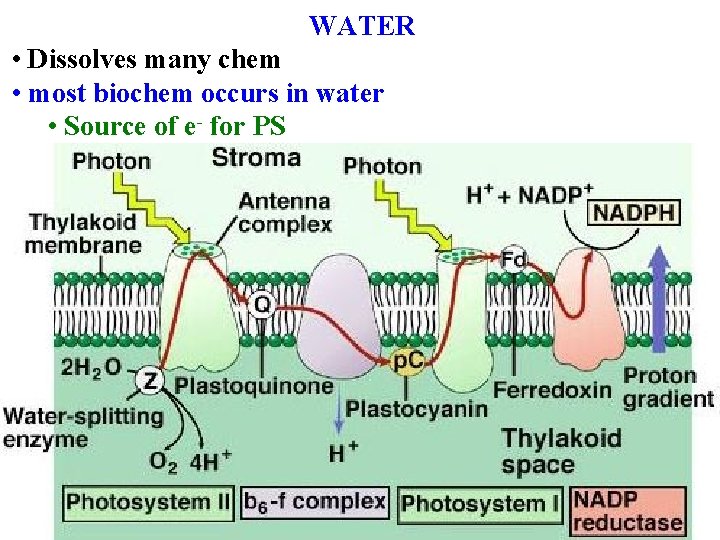
WATER • Plants' most important chemical • most often limits productivity • Often >90%% of a plant cell’s weight • Gives cells shape • Dissolves many chem

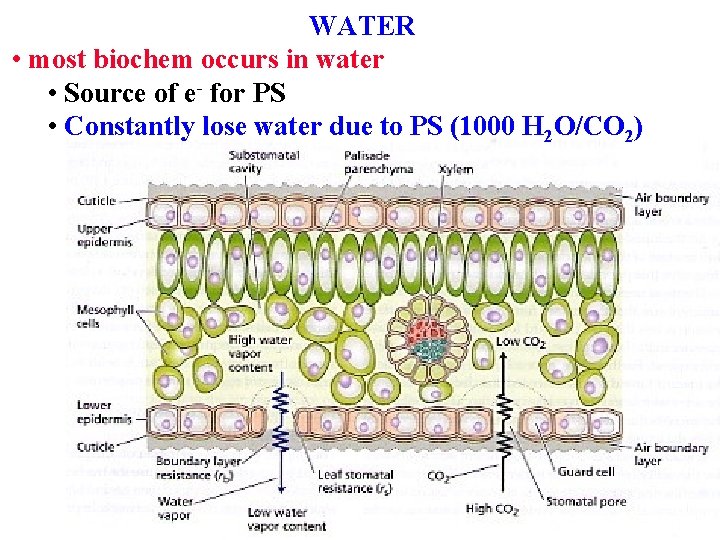
WATER • Dissolves many chem • most biochem occurs in water • Source of e- for PS

WATER • most biochem occurs in water • Source of e- for PS • Constantly lose water due to PS (1000 H 2 O/CO 2)

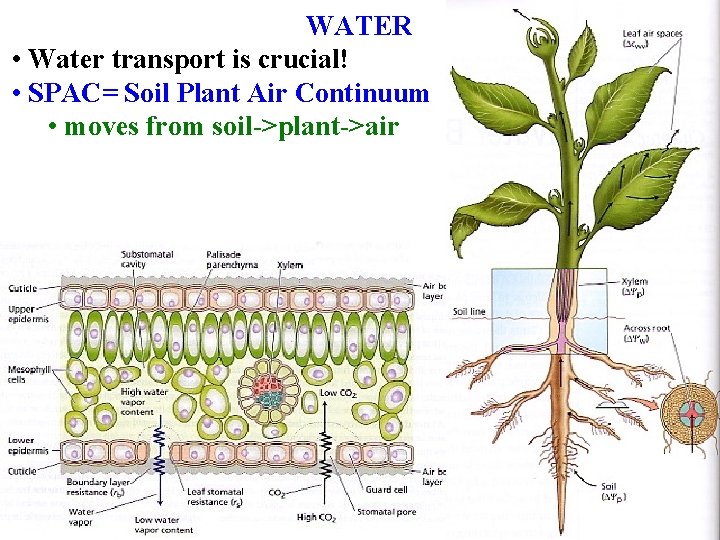
WATER • most biochem occurs in water • Source of e- for PS • Constantly lose water due to PS • Water transport is crucial!

WATER • Water transport is crucial! • SPAC= Soil Plant Air Continuum • moves from soil->plant->air

Plant Water Uptake Water is drawn through plants along the SPAC, using its special properties to draw it from the soil into the air

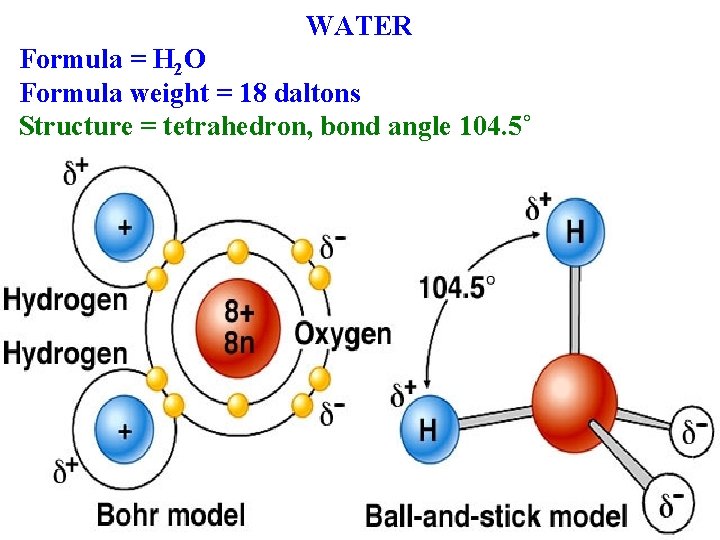
WATER Formula = H 2 O Formula weight = 18 daltons Structure = tetrahedron, bond angle 104. 5˚

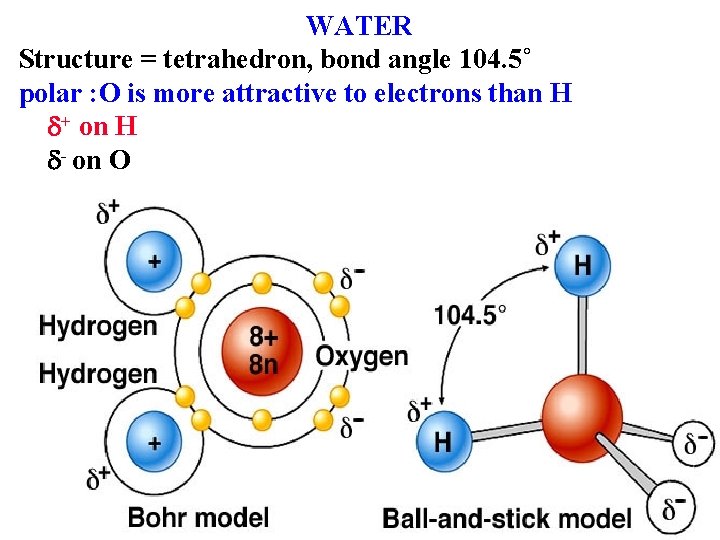
WATER Structure = tetrahedron, bond angle 104. 5˚ polar : O is more attractive to electrons than H + on H - on O

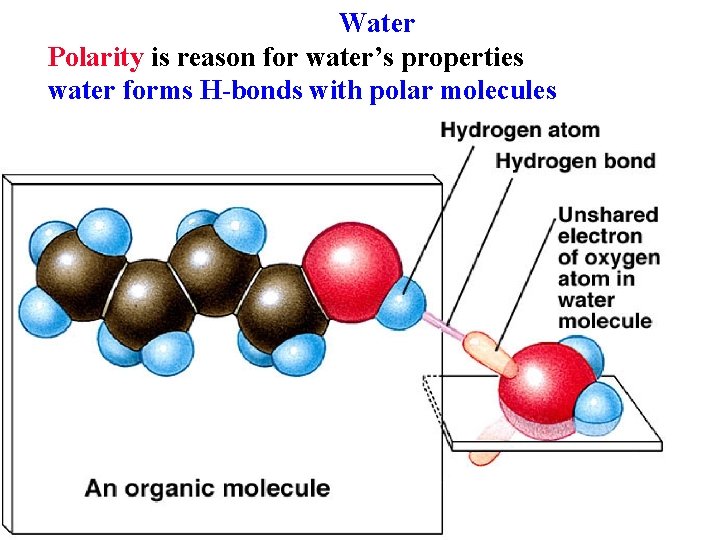
Water Polarity is reason for water’s properties water forms H-bonds with polar molecules

Water Polarity is reason for water’s properties water forms H-bonds with polar molecules Hydrophilic = polar molecules Hydrophobic = non-polar molecules

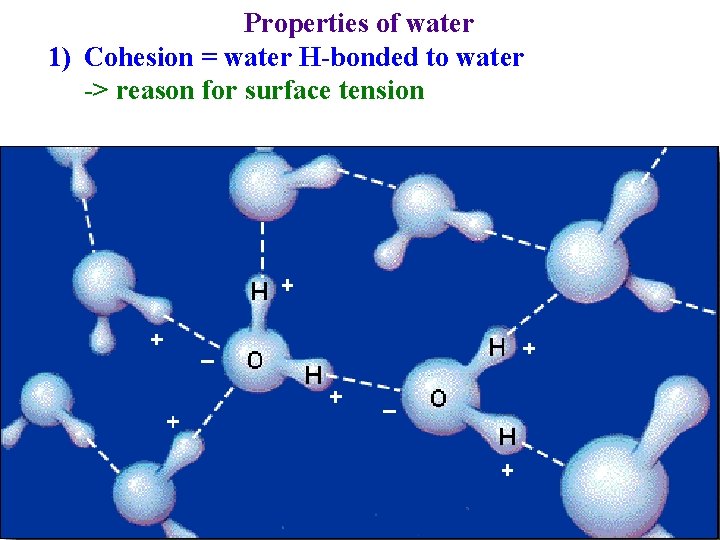
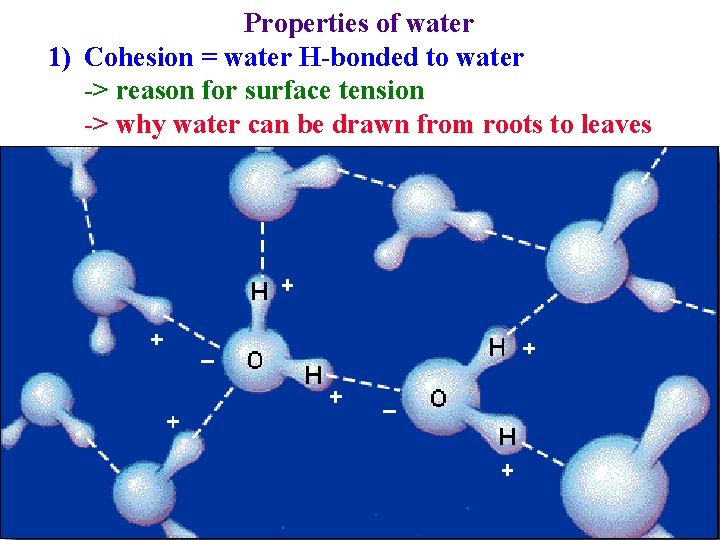
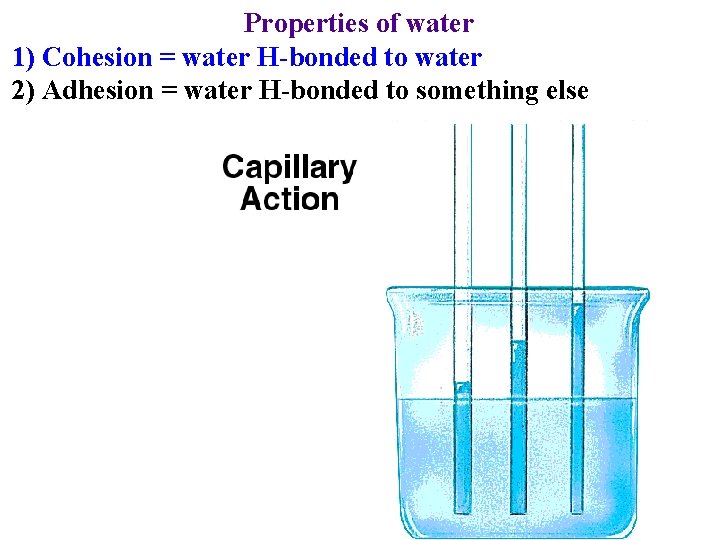
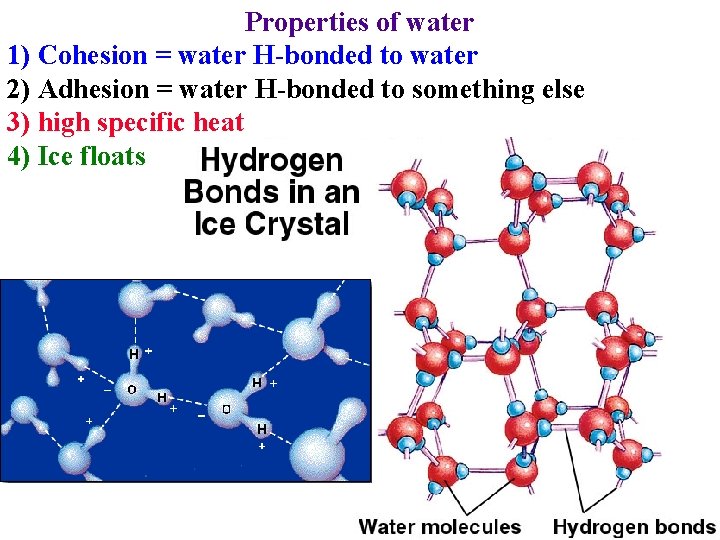
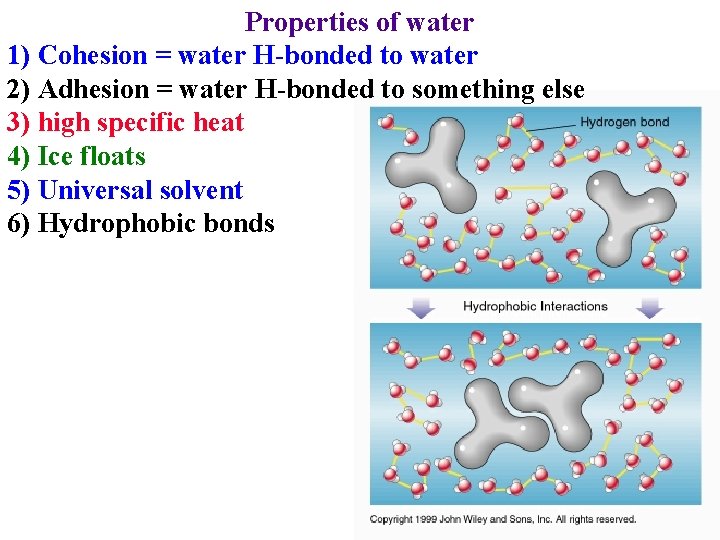
Properties of water 1) Cohesion = water H-bonded to water -> reason for surface tension

Properties of water 1) Cohesion = water H-bonded to water -> reason for surface tension -> why water can be drawn from roots to leaves

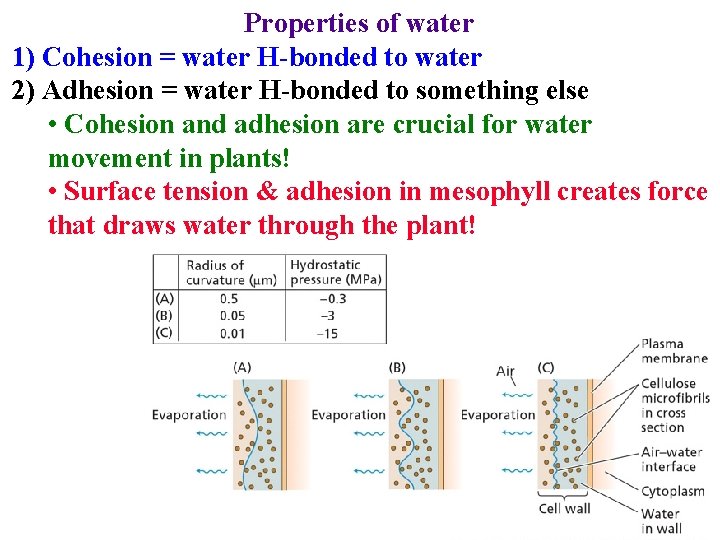
Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else

Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else • Cohesion and adhesion are crucial for water movement in plants!

Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else • Cohesion and adhesion are crucial for water movement in plants! • Surface tension & adhesion in mesophyll creates force that draws water through the plant!

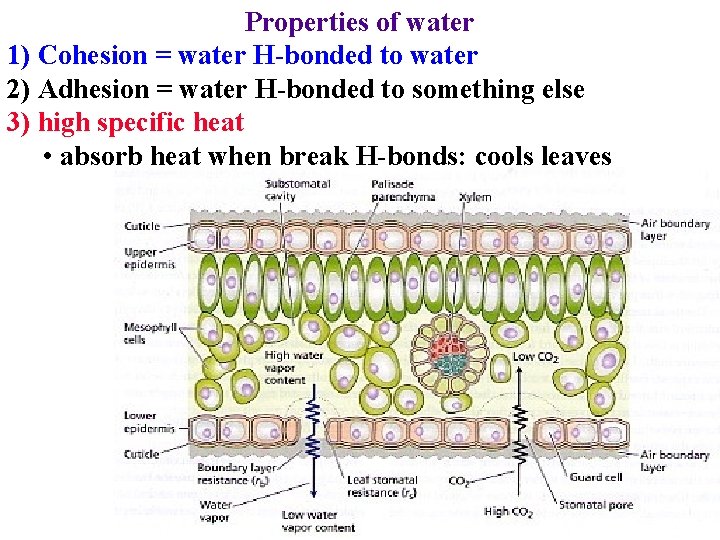
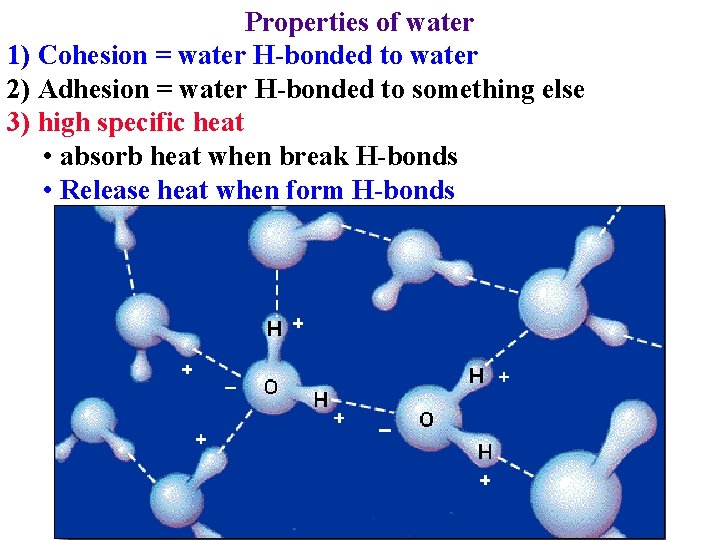
Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat • absorb heat when break H-bonds: cools leaves

Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat • absorb heat when break H-bonds • Release heat when form H-bonds

Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat 4) Ice floats

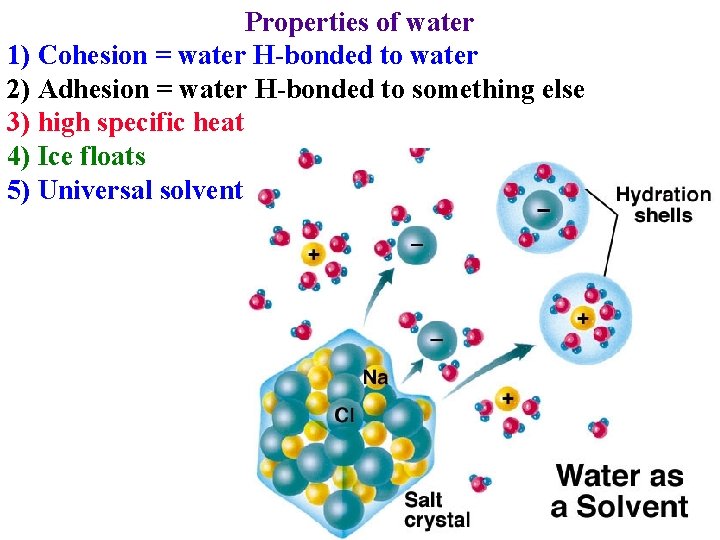
Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat 4) Ice floats 5) Universal solvent

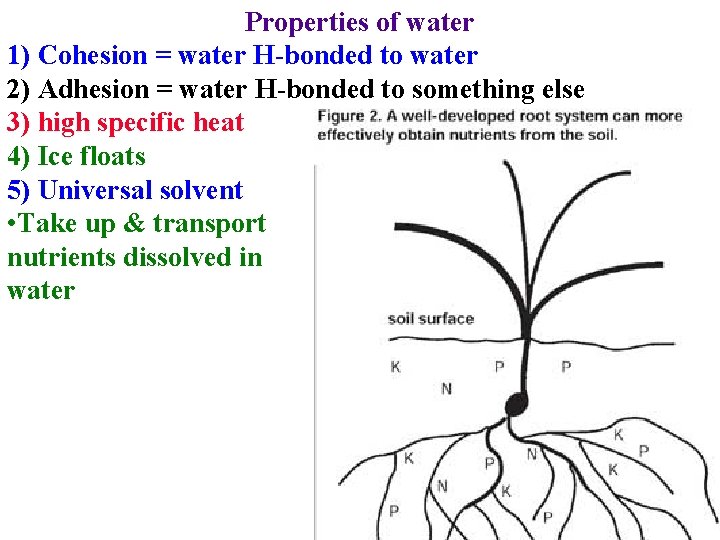
Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat 4) Ice floats 5) Universal solvent • Take up & transport nutrients dissolved in water

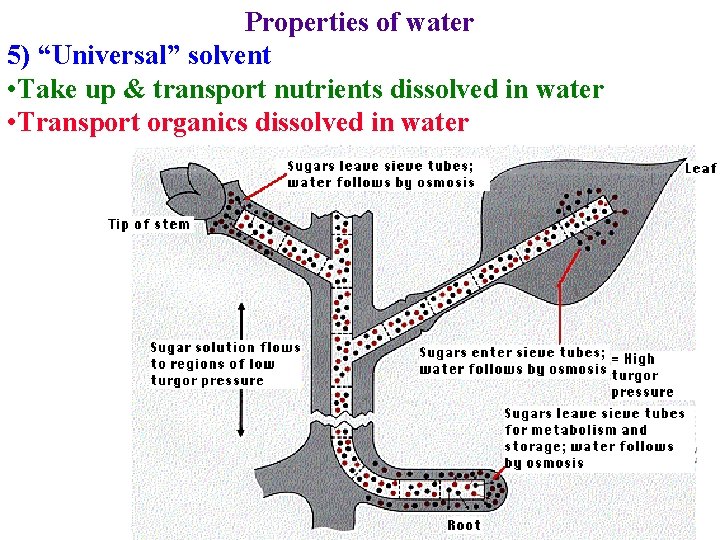
Properties of water 5) “Universal” solvent • Take up & transport nutrients dissolved in water • Transport organics dissolved in water

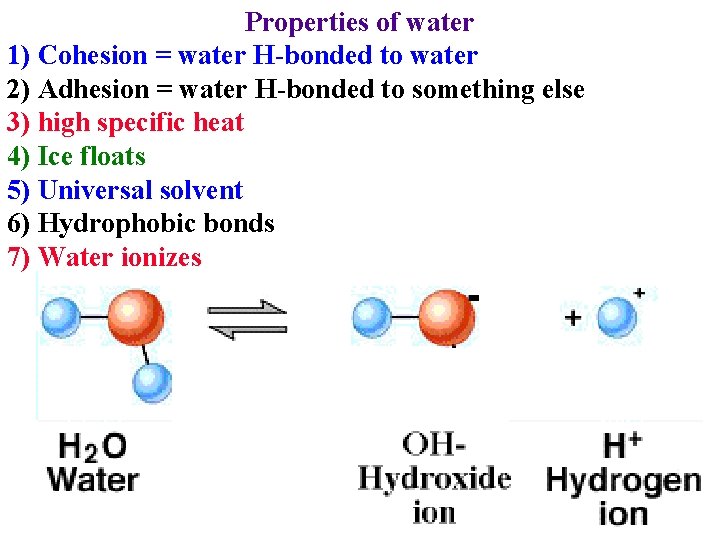
Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat 4) Ice floats 5) Universal solvent 6) Hydrophobic bonds

Properties of water 1) Cohesion = water H-bonded to water 2) Adhesion = water H-bonded to something else 3) high specific heat 4) Ice floats 5) Universal solvent 6) Hydrophobic bonds 7) Water ionizes
![p H H acidity of a solution p H convenient way to p. H [H+] = acidity of a solution p. H = convenient way to](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-27.jpg)
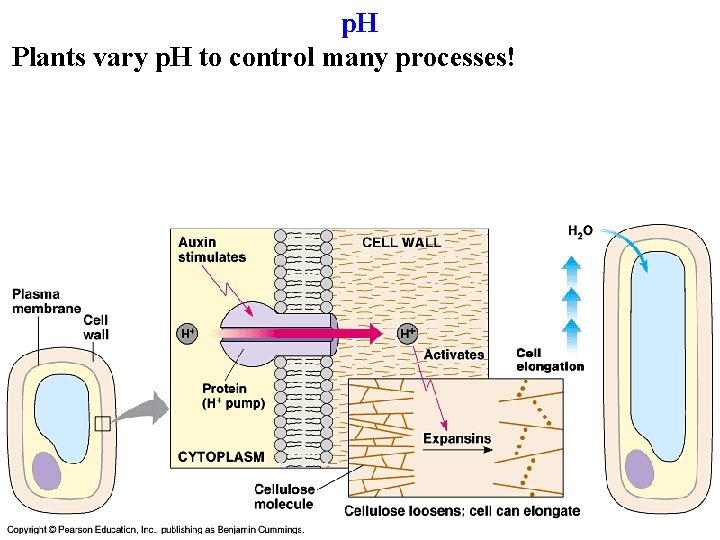
p. H [H+] = acidity of a solution p. H = convenient way to measure acidity p. H = - log 10 [H+] p. H 7 is neutral: [H+] = [OH-] -> at p. H 7 [H+] = 10 -7 moles/l

p. H Plants vary p. H to control many processes!
![Water movement Diffusion movement of single molecules down due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-29.jpg)
Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion until [ ] is even • Driving force?
![Water movement Diffusion movement of single molecules down due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-30.jpg)
Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion until [ ] is even • Driving force: lowers free energy • ∆G = ∆H- T∆S
![Water movement Diffusion movement of single molecules down due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-31.jpg)
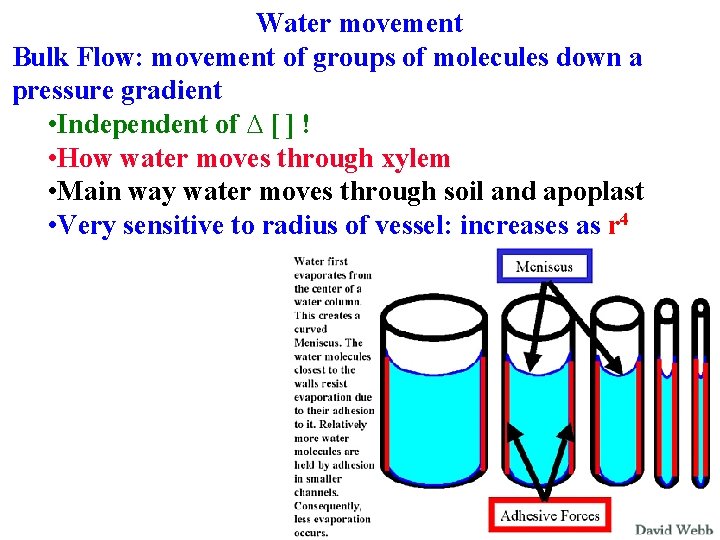
Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion until [ ] is even Bulk Flow: movement of groups of molecules down a pressure gradient
![Water movement Diffusion movement of single molecules down due to random motion Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-32.jpg)
Water movement Diffusion: movement of single molecules down ∆[ ] due to random motion until [ ] is even Bulk Flow: movement of groups of molecules down a pressure gradient • Independent of ∆ [ ] !
![Water movement Diffusion movement of single molecules down due to random motion until Water movement Diffusion: movement of single molecules down ∆[] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-33.jpg)
Water movement Diffusion: movement of single molecules down ∆[] due to random motion until [ ] is even Bulk Flow: movement of groups of molecules down a pressure gradient • Independent of ∆[ ] ! • How water moves through xylem
![Water movement Diffusion movement of single molecules down due to random motion until Water movement Diffusion: movement of single molecules down [] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-34.jpg)
Water movement Diffusion: movement of single molecules down [] due to random motion until [ ] is even Bulk Flow: movement of groups of molecules down a pressure gradient • Independent of ∆ [ ] ! • How water moves through xylem • How water moves through soil and apoplast

Water movement Bulk Flow: movement of groups of molecules down a pressure gradient • Independent of ∆ [ ] ! • How water moves through xylem • Main way water moves through soil and apoplast • Very sensitive to radius of vessel: increases as r 4
![Water movement Diffusion movement of single molecules down due to random motion until Water movement Diffusion: movement of single molecules down ∆[] due to random motion until](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-36.jpg)
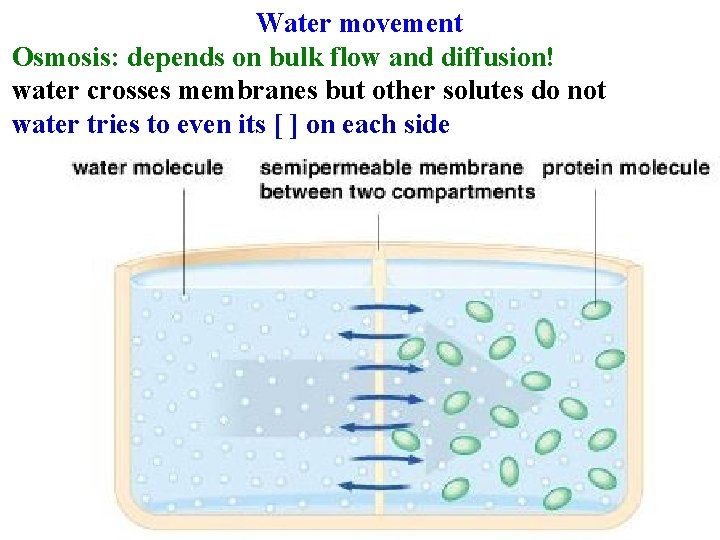
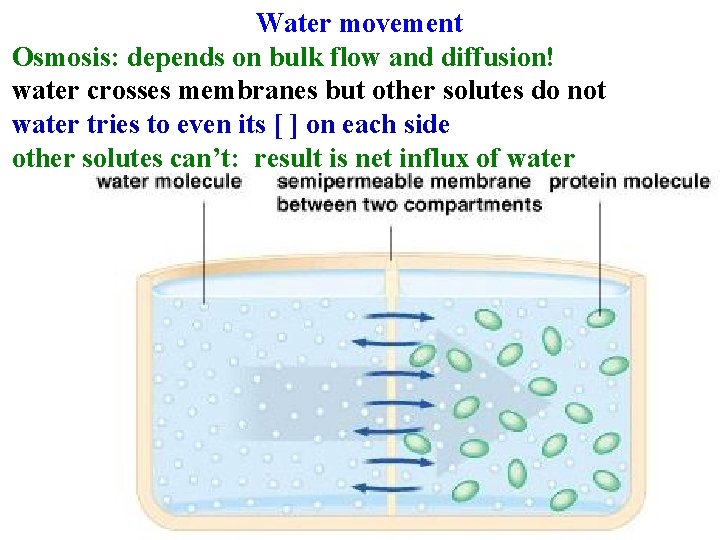
Water movement Diffusion: movement of single molecules down ∆[] due to random motion until [ ] is even Bulk Flow: movement of groups of molecules down a pressure gradient • Independent of ∆[ ] ! • How water moves through xylem • Main way water moves through soil and apoplast • Very sensitive to radius of vessel: increases as r 4 Osmosis: depends on bulk flow and diffusion!

Water movement Osmosis: depends on bulk flow and diffusion! water crosses membranes but other solutes do not water tries to even its [ ] on each side

Water movement Osmosis: depends on bulk flow and diffusion! water crosses membranes but other solutes do not water tries to even its [ ] on each side other solutes can’t: result is net influx of water

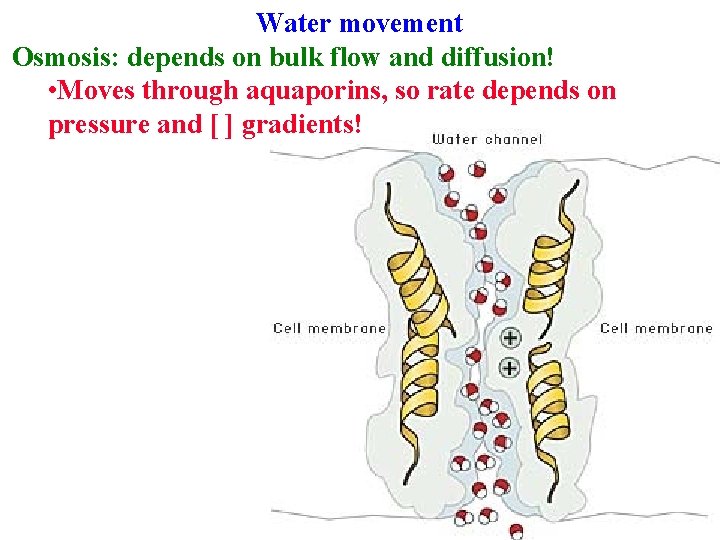
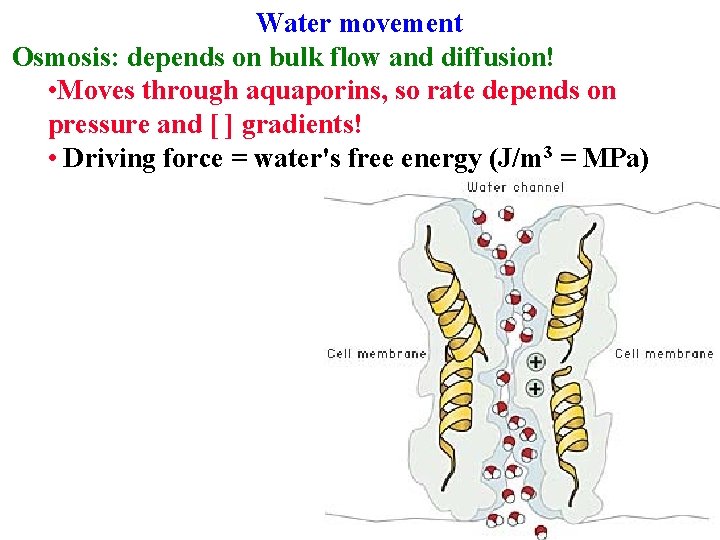
Water movement Osmosis: depends on bulk flow and diffusion! • Moves through aquaporins, so rate depends on pressure and [ ] gradients!

Water movement Osmosis: depends on bulk flow and diffusion! • Moves through aquaporins, so rate depends on pressure and [ ] gradients! • Driving force = water's free energy (J/m 3 = MPa)

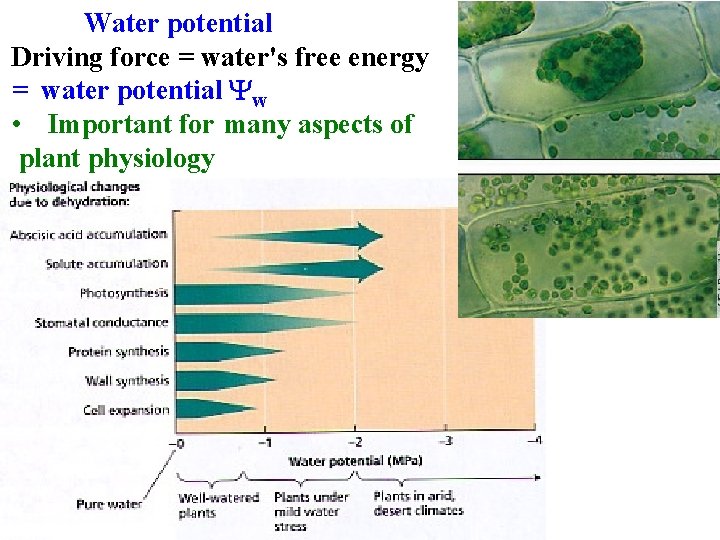
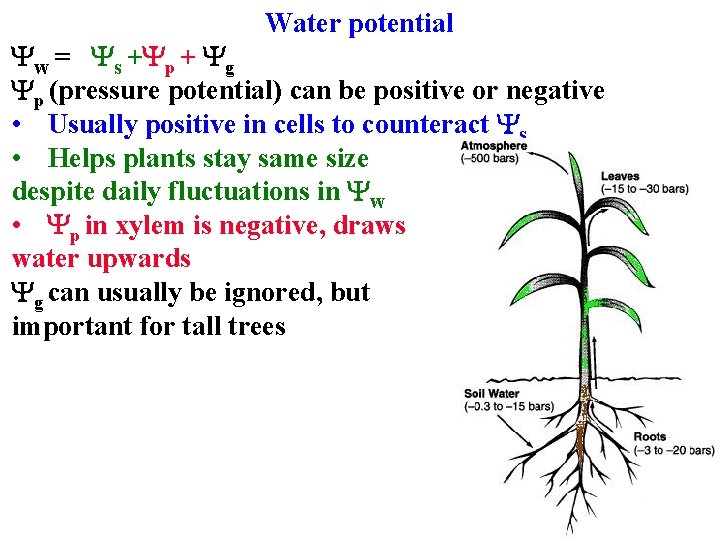
Water potential Driving force = water's free energy = water potential Yw • Important for many aspects of plant physiology

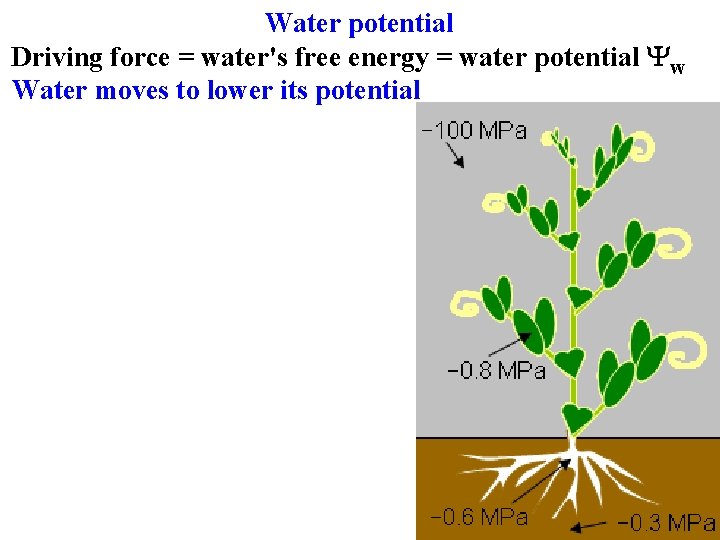
Water potential Driving force = water's free energy = water potential Yw Water moves to lower its potential

Water potential Driving force = water's free energy = water potential Yw Water moves to lower its potential

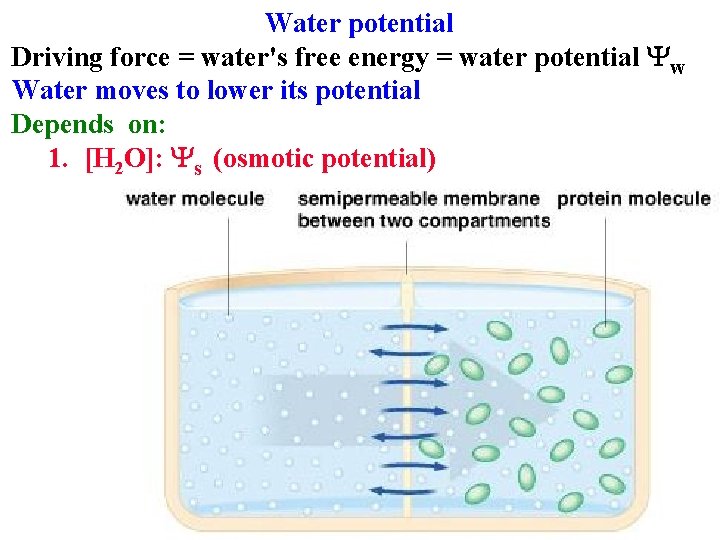
Water potential Driving force = water's free energy = water potential Yw Water moves to lower its potential Depends on: 1. [H 2 O]: Ys (osmotic potential)
![Water potential Water moves to lower its potential Depends on 1 H 2 O Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-45.jpg)
Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Ys (osmotic potential) 2. Pressure : Yp • Turgor pressure inside cells
![Water potential Water moves to lower its potential Depends on 1 H 2 O Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-46.jpg)
Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Ys (osmotic potential) 2. Pressure : Yp • Turgor pressure inside cells • Negative pressure in xylem!
![Water potential Water moves to lower its potential Depends on 1 H 2 O Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-47.jpg)
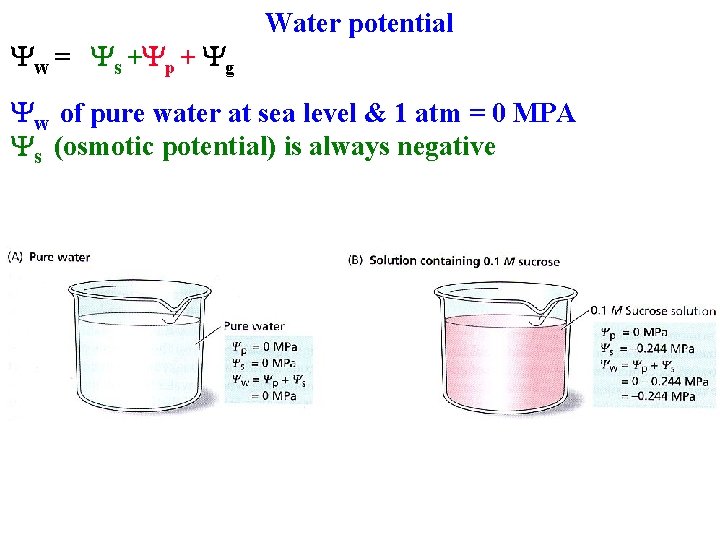
Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Ys (osmotic potential) 2. Pressure Yp 3. Gravity Yg 4. Yw = Ys +Yp + Yg
![Water potential Water moves to lower its potential Depends on 1 H 2 O Water potential Water moves to lower its potential Depends on: 1. [H 2 O]:](https://slidetodoc.com/presentation_image_h2/4c6d0638af43969b8098334040623c3f/image-48.jpg)
Water potential Water moves to lower its potential Depends on: 1. [H 2 O]: Ys (osmotic potential) 2. Pressure Yp 3. Gravity Yg 4. Yw = Ys +Yp + Yg 5. Yw of pure water at sea level 6. & 1 atm = 0 MPA

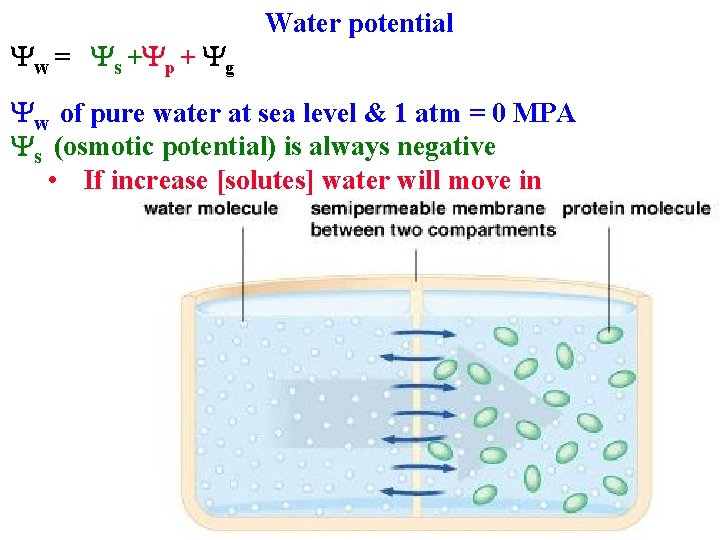
Yw = Ys +Yp + Yg Water potential Yw of pure water at sea level & 1 atm = 0 MPA Ys (osmotic potential) is always negative

Yw = Ys +Yp + Yg Water potential Yw of pure water at sea level & 1 atm = 0 MPA Ys (osmotic potential) is always negative • If increase [solutes] water will move in

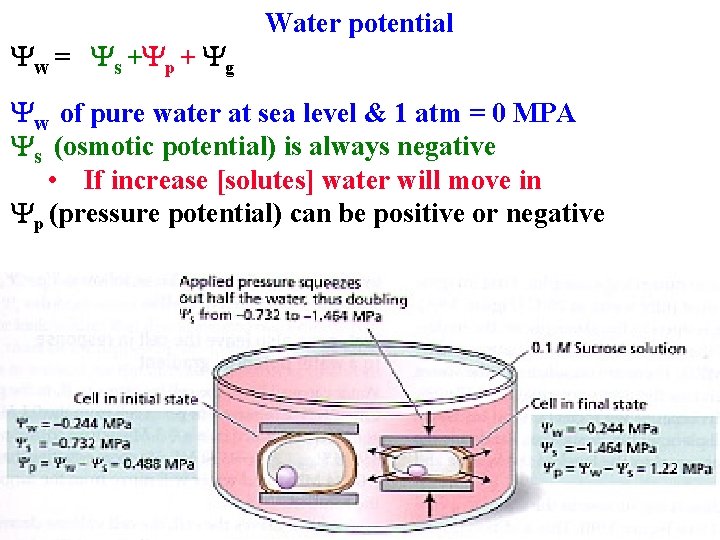
Yw = Ys +Yp + Yg Water potential Yw of pure water at sea level & 1 atm = 0 MPA Ys (osmotic potential) is always negative • If increase [solutes] water will move in Yp (pressure potential) can be positive or negative

Yw = Ys +Yp + Yg Water potential Yw of pure water at sea level & 1 atm = 0 MPA Ys (osmotic potential) is always negative • If increase [solutes] water will move in Yp (pressure potential) can be positive or negative • Usually positive in cells to counteract Ys

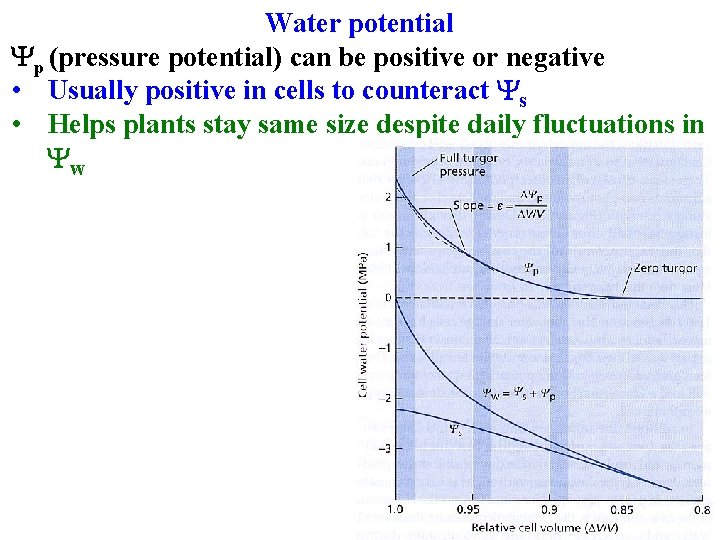
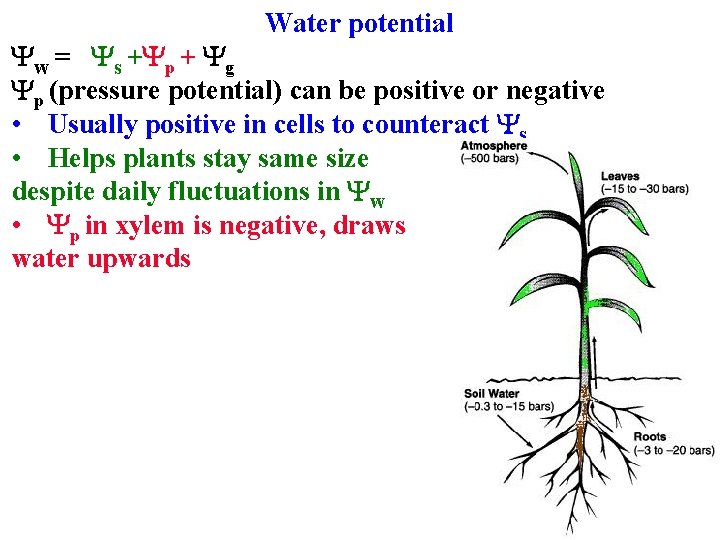
Water potential Yp (pressure potential) can be positive or negative • Usually positive in cells to counteract Ys • Helps plants stay same size despite daily fluctuations in Yw

Water potential Yw = Ys +Yp + Yg Yp (pressure potential) can be positive or negative • Usually positive in cells to counteract Ys • Helps plants stay same size despite daily fluctuations in Yw • Yp in xylem is negative, draws water upwards

Water potential Yw = Ys +Yp + Yg Yp (pressure potential) can be positive or negative • Usually positive in cells to counteract Ys • Helps plants stay same size despite daily fluctuations in Yw • Yp in xylem is negative, draws water upwards Yg can usually be ignored, but important for tall trees

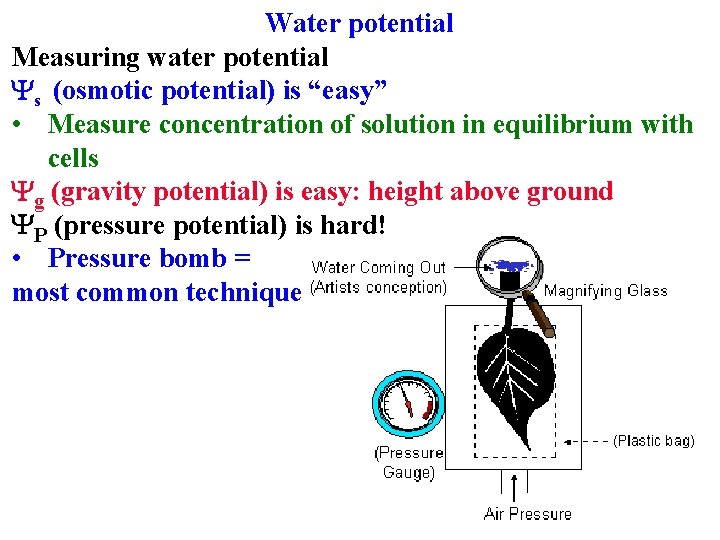
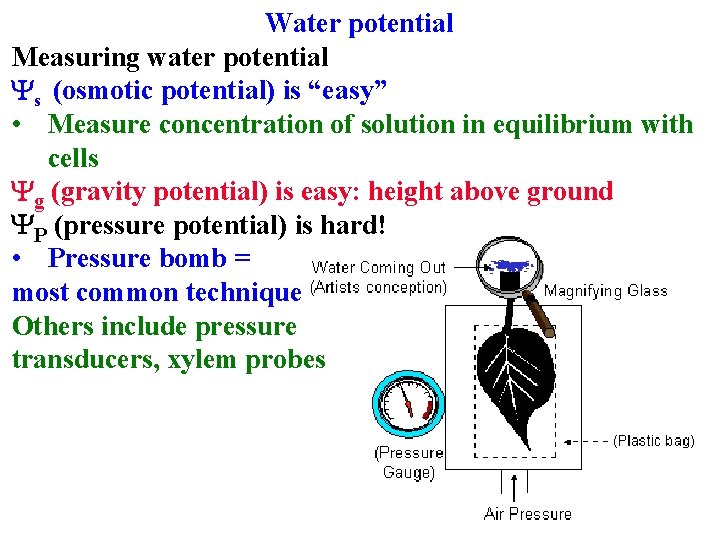
Water potential Measuring water potential

Water potential Measuring water potential Ys (osmotic potential) is “easy” • Measure concentration of solution in equilibrium with cells

Water potential Measuring water potential Ys (osmotic potential) is “easy” • Measure concentration of solution in equilibrium with cells Yg (gravity potential) is easy: height above ground • -0. 01 Mpa/m

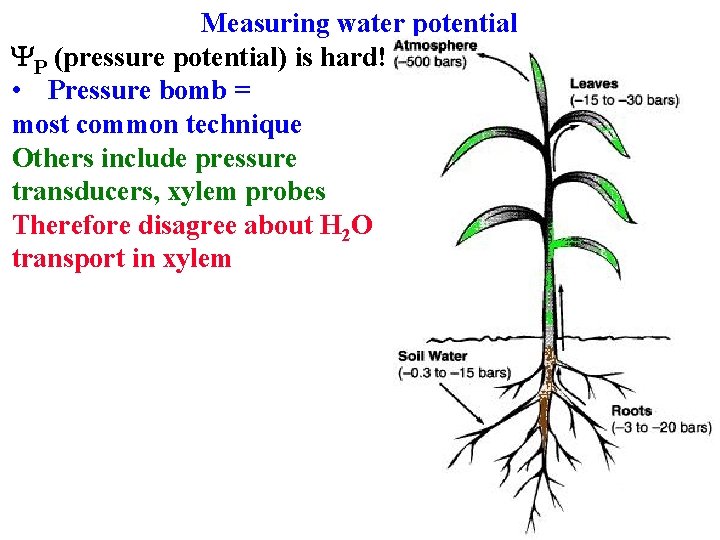
Water potential Measuring water potential Ys (osmotic potential) is “easy” • Measure concentration of solution in equilibrium with cells Yg (gravity potential) is easy: height above ground YP (pressure potential) is hard! • Pressure bomb = most common technique

Water potential Measuring water potential Ys (osmotic potential) is “easy” • Measure concentration of solution in equilibrium with cells Yg (gravity potential) is easy: height above ground YP (pressure potential) is hard! • Pressure bomb = most common technique Others include pressure transducers, xylem probes

Measuring water potential YP (pressure potential) is hard! • Pressure bomb = most common technique Others include pressure transducers, xylem probes Therefore disagree about H 2 O transport in xylem