Water PH and buffer Specific properties of water
Water, PH and buffer Specific properties of water Hydrogen binding, Acidity, H+, PH and buffer


Biochemistry l l l l Biochemistry is the Chemistry of life(Gk , bios “life” Area for study: cell biology, molecular biology and molecular genetics Aim of Biochemistry: Describe and explain , in molecular terms , all chemical process of living cells( exm. contractibility in muscle cells) How life began Scope of biochemistry Classification Medical biochemistry • • General biochemistry Clinical biochemistry
Water, PH and buffer l l l l Interrelationship of biochemistry and medicine Two – way street(structure and function: normal and sickle cell Hb, PKU, alkaptonuria, …. ) Health All disease has a biochemical basis Treatment of disease Inborn error of disease
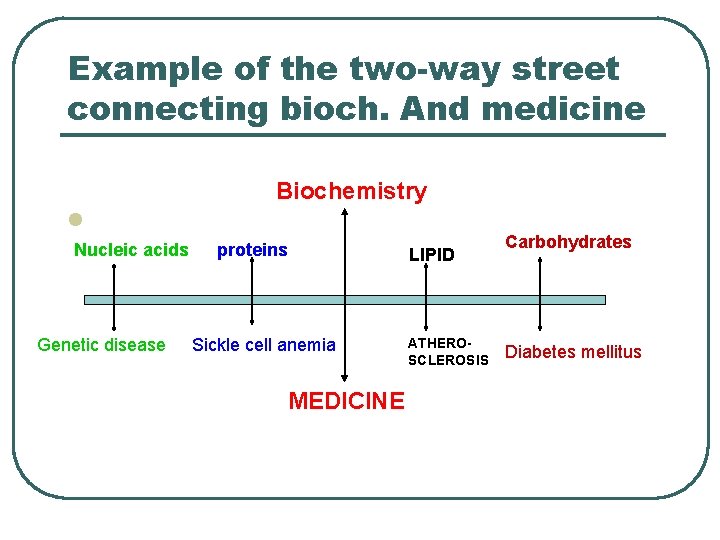
Example of the two-way street connecting bioch. And medicine Biochemistry l Nucleic acids Genetic disease proteins Sickle cell anemia MEDICINE LIPID ATHEROSCLEROSIS Carbohydrates Diabetes mellitus
Water, PH and buffer l l l Normal biochemical processes are the basis of health WHO and define of health Biochemical research has impact on nutrition and preventive medicine
A knowledge of Biochemistry is essential to all life sciences l l l Genetic, Physiology Immunology Pharmacology& pharmacy Toxicology Pathology, Microbiology, Zoology, Botany RESUT; Biochemistry as common language
Human genome project(HGP( l l l Sequencing of human genome(1990, 2000) International human genome sequencing consortium and Celera Genomics This project completed in 2003 (after 50 ys discovery of ds DNA)
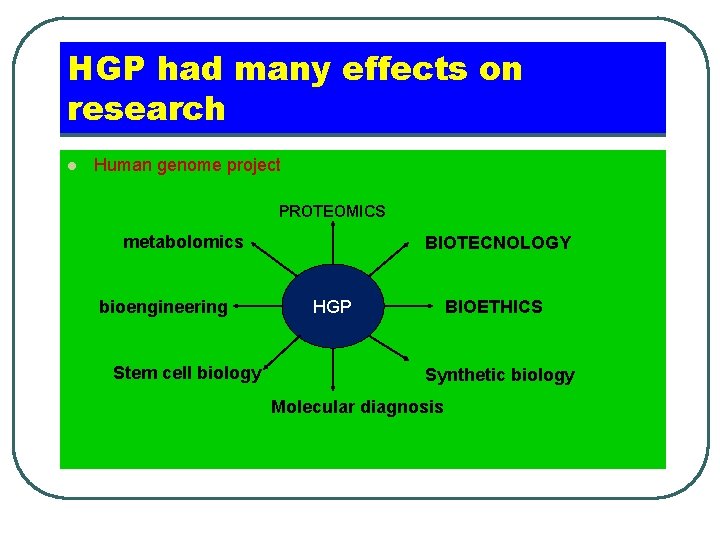
HGP had many effects on research l Human genome project PROTEOMICS metabolomics bioengineering Stem cell biology BIOTECNOLOGY HGP BIOETHICS Synthetic biology Molecular diagnosis
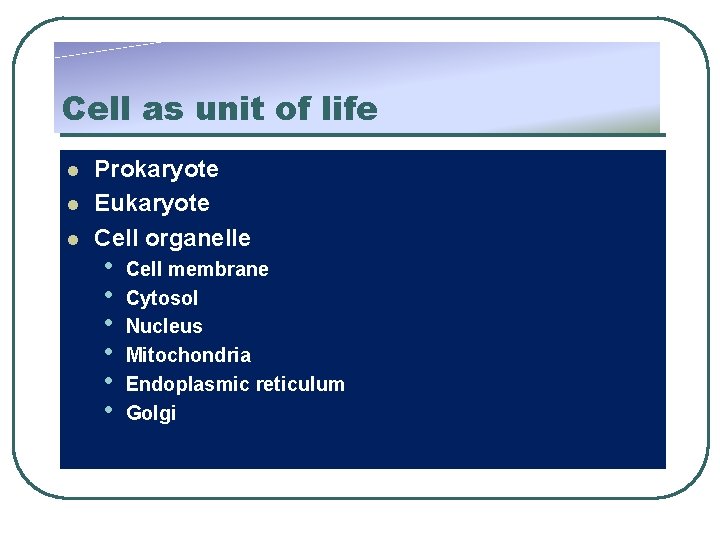
Cell as unit of life l l l Prokaryote Eukaryote Cell organelle • • • Cell membrane Cytosol Nucleus Mitochondria Endoplasmic reticulum Golgi
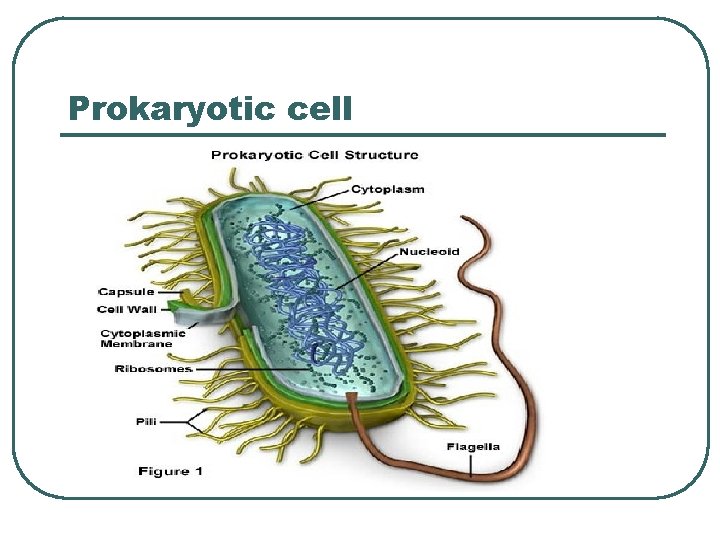
Prokaryotic cell
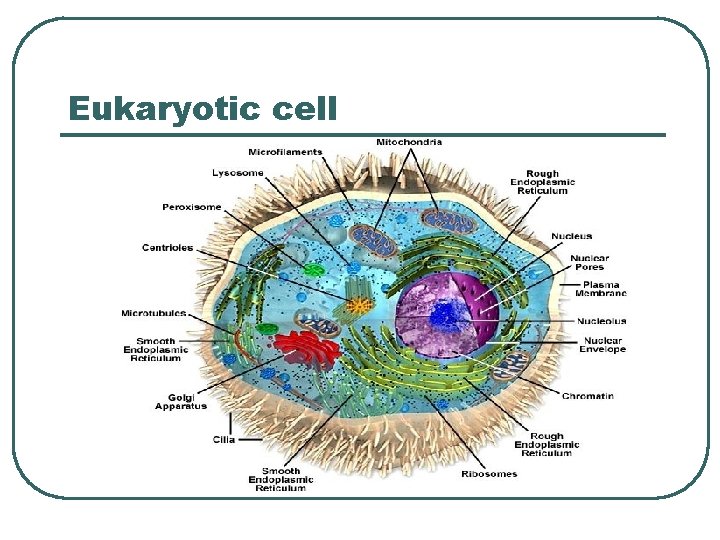
Eukaryotic cell
Cell fractionation l l Cell suspension (Blender) Cell homogenization (Poter) Cell extract Centrifugation • Density gradient • Sequential sedimentation • Sequential floatation
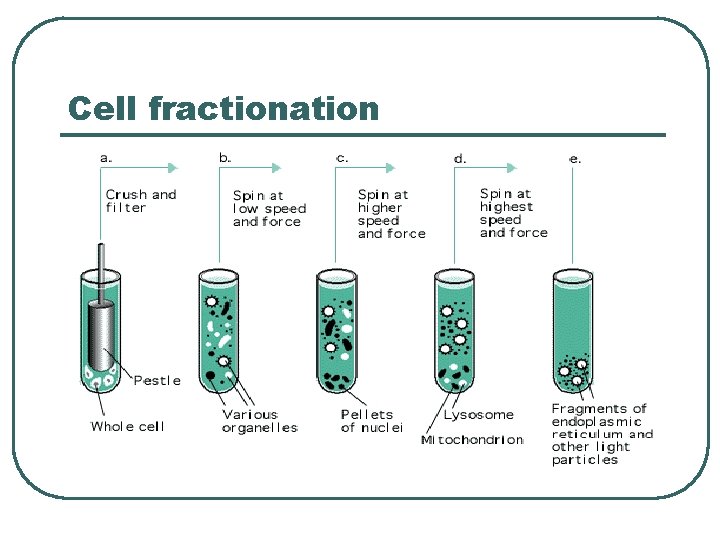
Cell fractionation
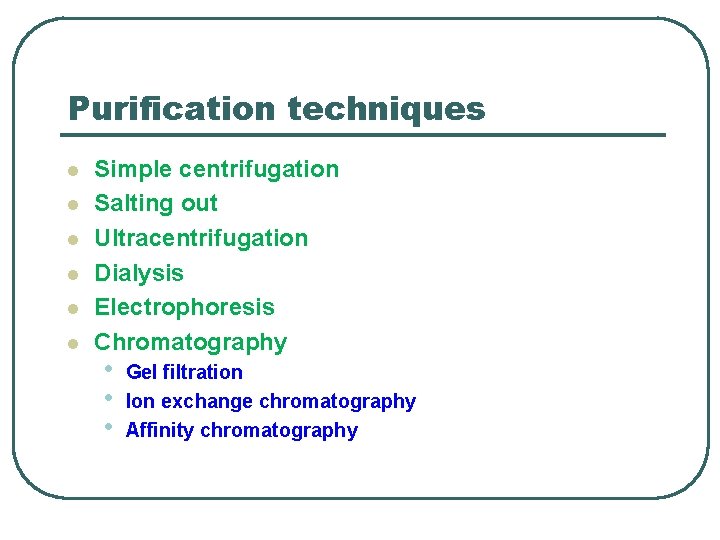
Purification techniques l l l Simple centrifugation Salting out Ultracentrifugation Dialysis Electrophoresis Chromatography • • • Gel filtration Ion exchange chromatography Affinity chromatography
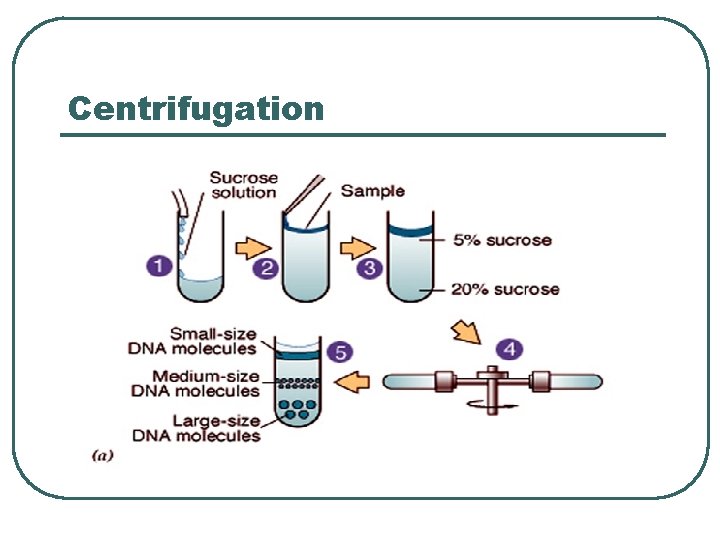
Centrifugation
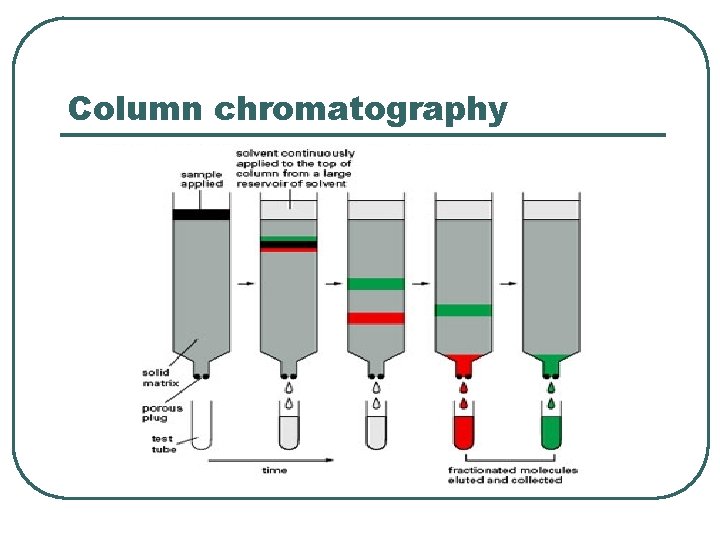
Column chromatography
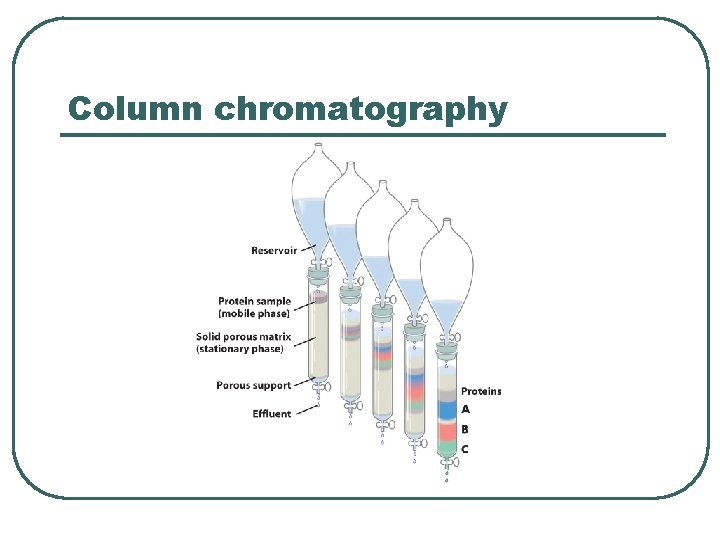
Column chromatography

Paper chromatography
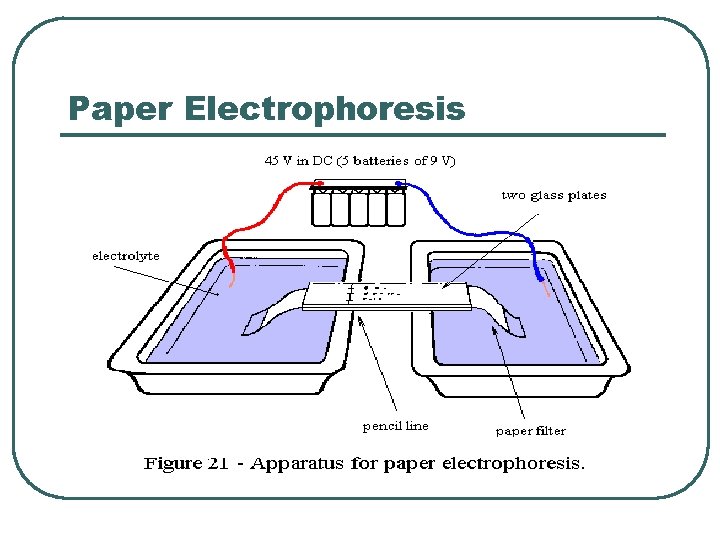
Paper Electrophoresis
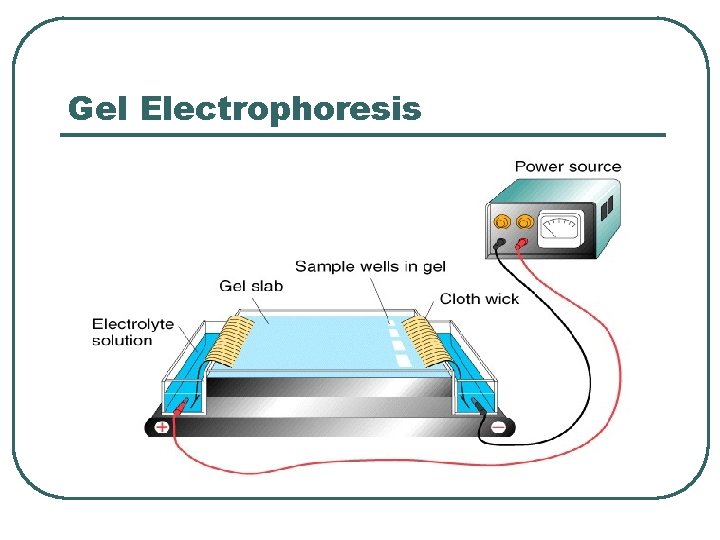
Gel Electrophoresis
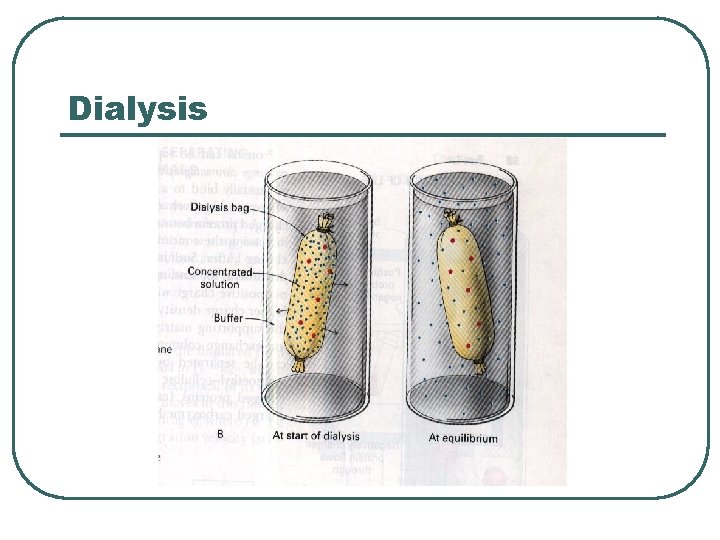
Dialysis
Water and p. H l l l l TWO- THIRD OF TOTAL BODY WEIGHT(55 -65%) IN MEN AND 10% LESS IN WOMEN It is distributed in intracellular and extracellular fluid Regulation of water balance depends on hypothalamic mechanism ADH(2% increase in extracellular fluid osmolarity) Kidney And others Non osmotic mechanism(10% decrease in extracellular volume Physiologic p. H and buffer systems( bicarbonate, phosphate and proteins)
Special properties of water l Water, the most vital molecule • High melting point • High boiling point • High thermal content • High dielectric constant • ……………. .
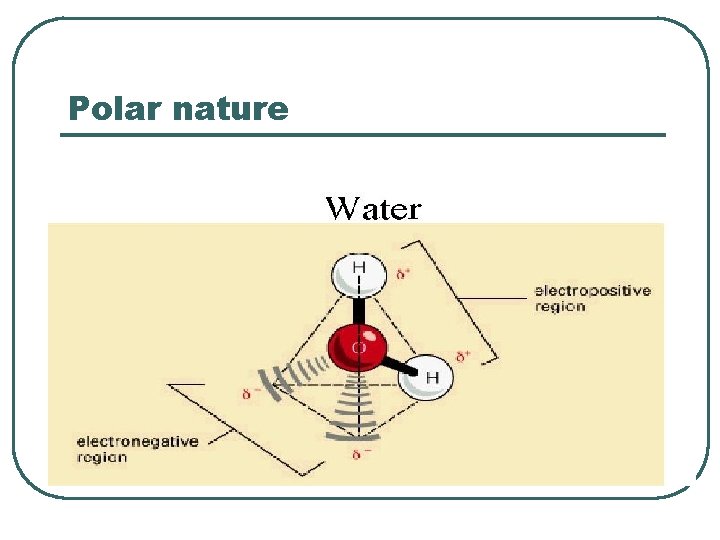
Polar nature
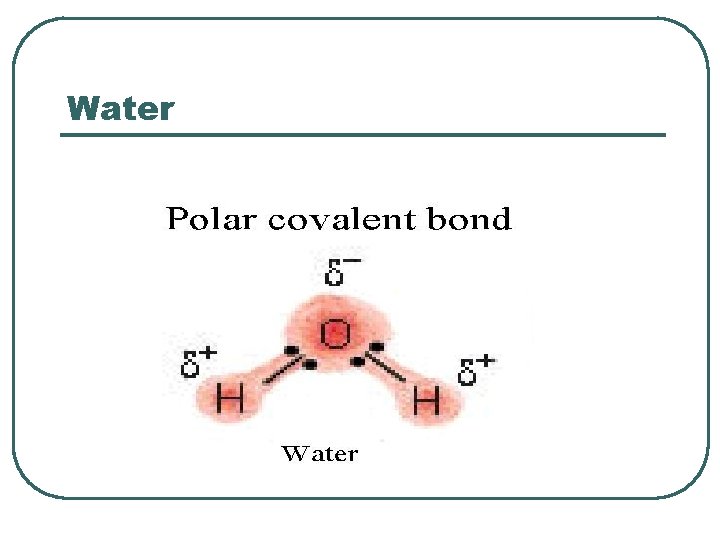
Water
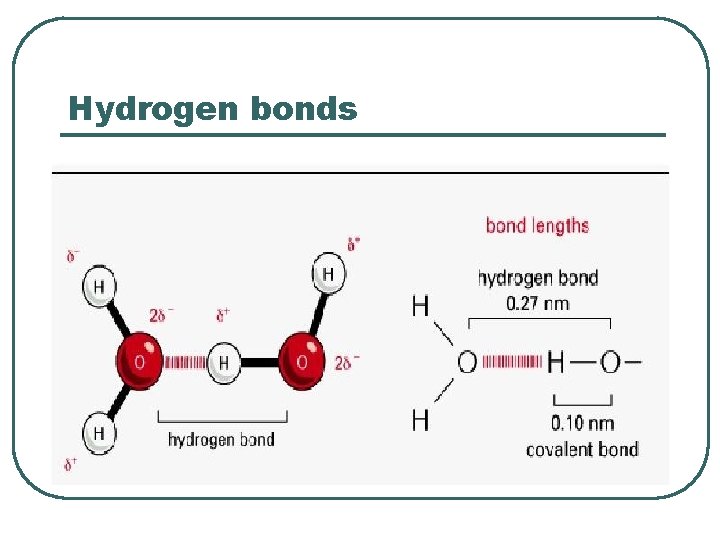
Hydrogen bonds
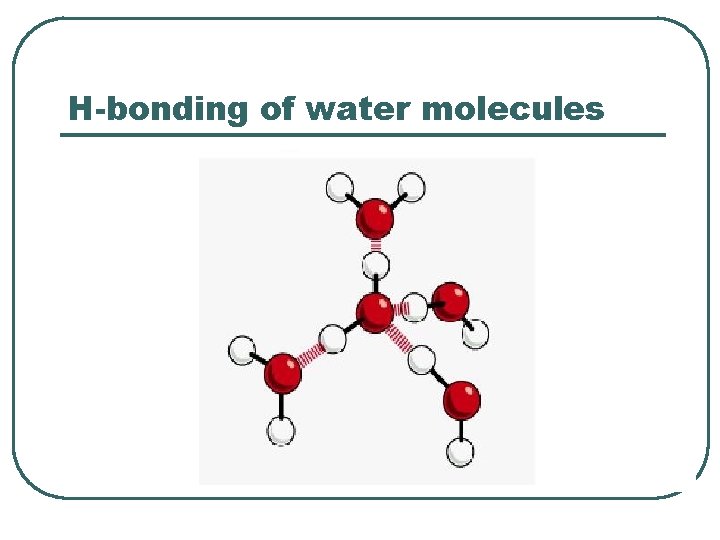
H-bonding of water molecules
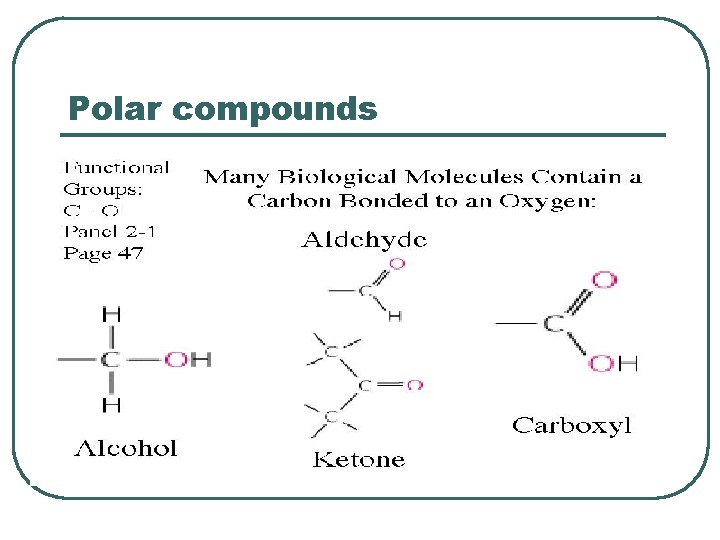
Polar compounds
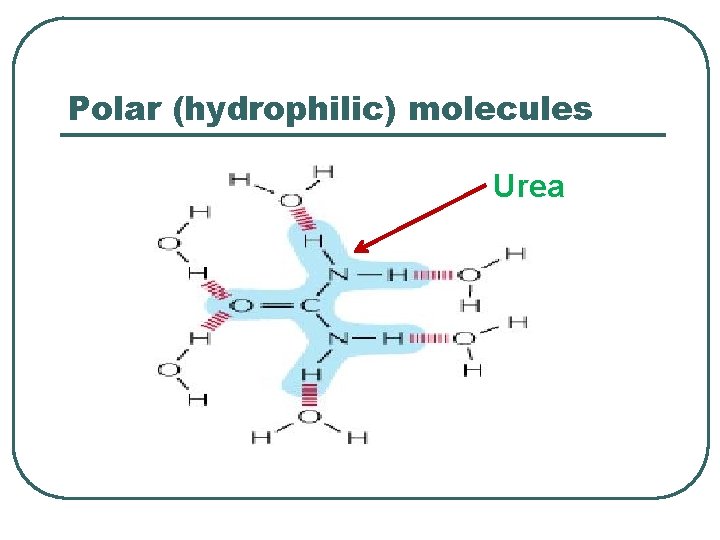
Polar (hydrophilic) molecules Urea
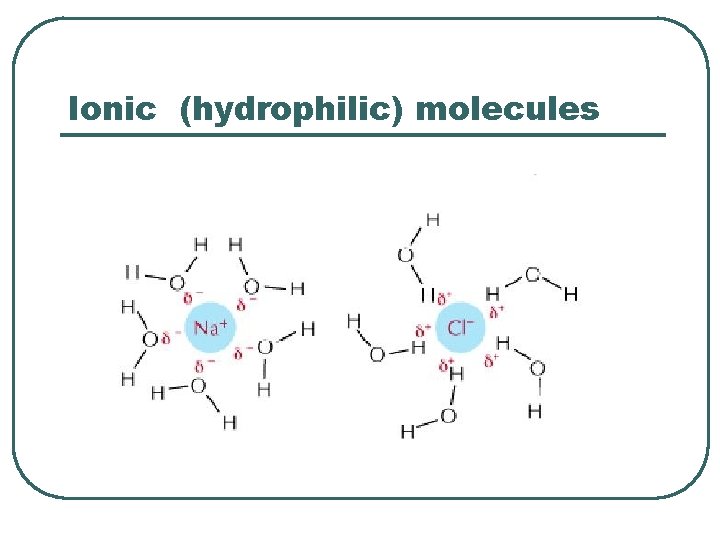
Ionic (hydrophilic) molecules
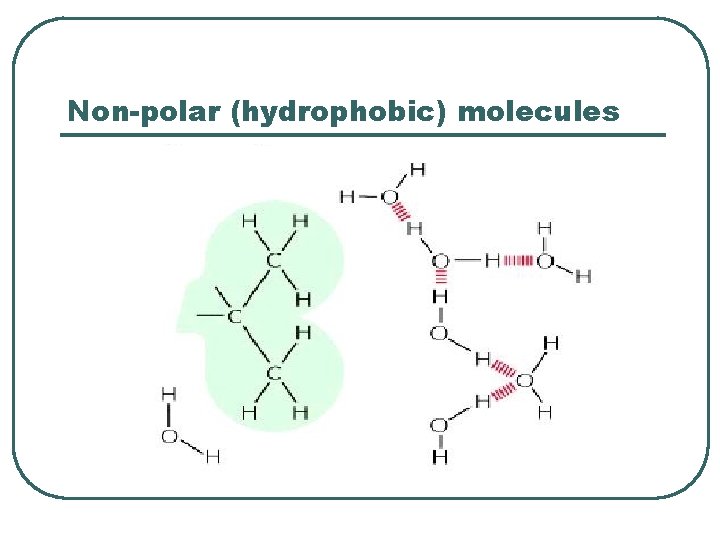
Non-polar (hydrophobic) molecules

Water ionization
![Ionization of water H 2 O ↔ H+ + OHK= [H+] [OH-] / [H Ionization of water H 2 O ↔ H+ + OHK= [H+] [OH-] / [H](http://slidetodoc.com/presentation_image_h2/57bd305e3fd3368f7bcca146e273a80e/image-35.jpg)
Ionization of water H 2 O ↔ H+ + OHK= [H+] [OH-] / [H 2 O] = 1. 8 × 10 -16 K= [10 -7]/[55. 56]=1. 8 x 10 -16 mol/L Kw =(K)[H 2 O]= [H+] [OH-] = 1 × 10 -14 (1. 8 x 10 -16 mol/L)(55. 56)=1 X 10 -14(mol/l)2 PH = -log [H+] PH + POH = 14
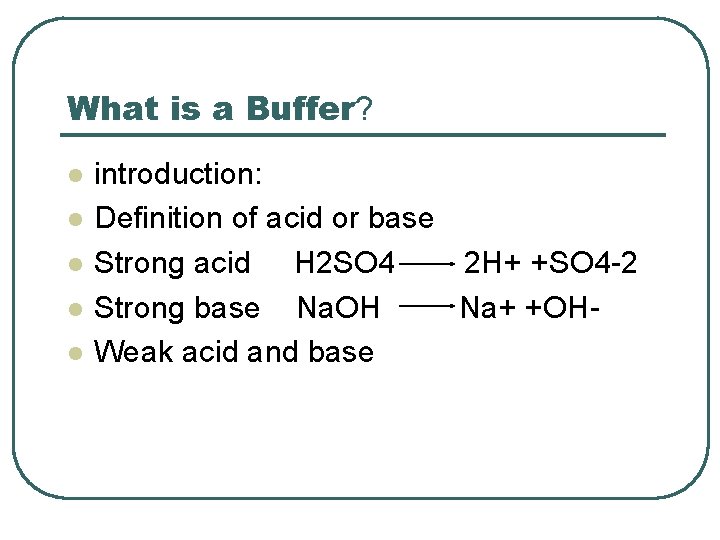
What is a Buffer? l l l introduction: Definition of acid or base Strong acid H 2 SO 4 2 H+ +SO 4 -2 Strong base Na. OH Na+ +OHWeak acid and base
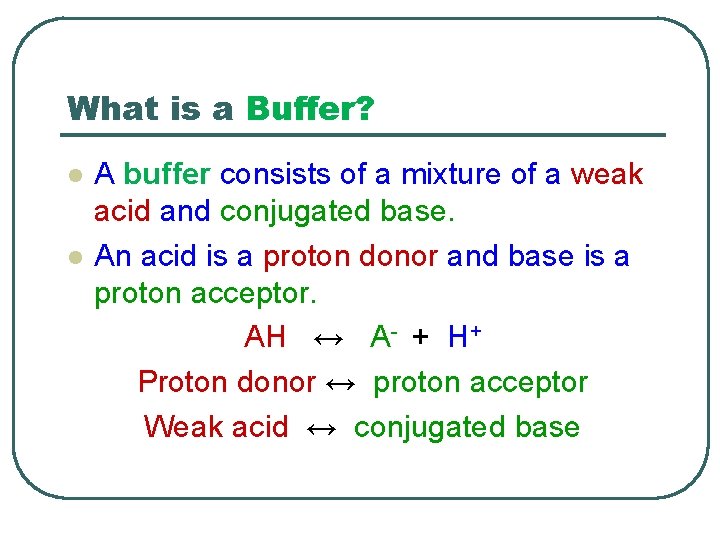
What is a Buffer? l l A buffer consists of a mixture of a weak acid and conjugated base. An acid is a proton donor and base is a proton acceptor. AH ↔ A- + H+ Proton donor ↔ proton acceptor Weak acid ↔ conjugated base
![Henderson – Haselbach equation l l l l HA A- + H+ Ka =[A-][H+]/[HA] Henderson – Haselbach equation l l l l HA A- + H+ Ka =[A-][H+]/[HA]](http://slidetodoc.com/presentation_image_h2/57bd305e3fd3368f7bcca146e273a80e/image-38.jpg)
Henderson – Haselbach equation l l l l HA A- + H+ Ka =[A-][H+]/[HA] [H+]=Ka[HA]/[A-] Log[H+]=log(ka [HA]/[A-]) Log[H+]=logka+log[HA]/[A-] -Log[H+]=-logka-log[HA]/[A-] p. H=p. Ka+log[A-]/[HA]
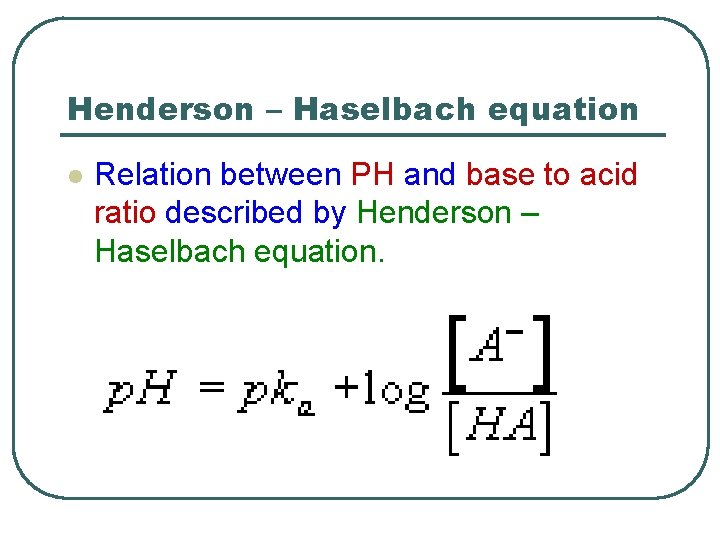
Henderson – Haselbach equation l Relation between PH and base to acid ratio described by Henderson – Haselbach equation.

Buffer capacity l Buffer systems tends to be most effective when the PH is equal to their weak acid PKa. (a range PKa± 1) l More concentrated buffers are more effective.
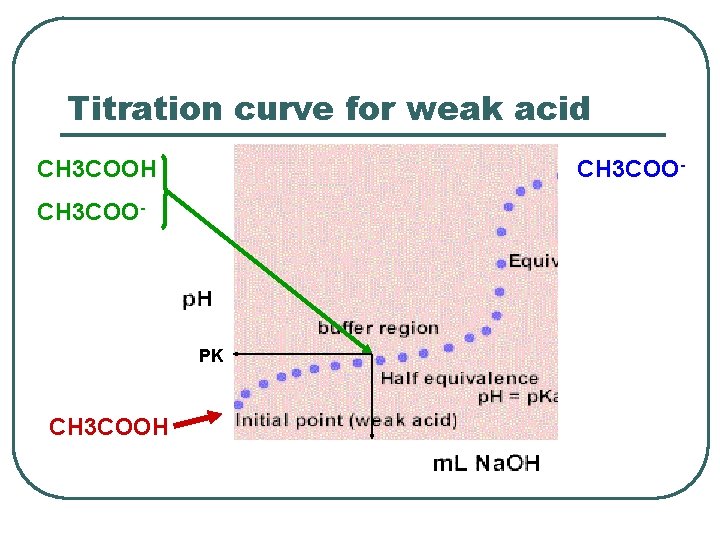
Titration curve for weak acid CH 3 COOH CH 3 COO- PK CH 3 COOH
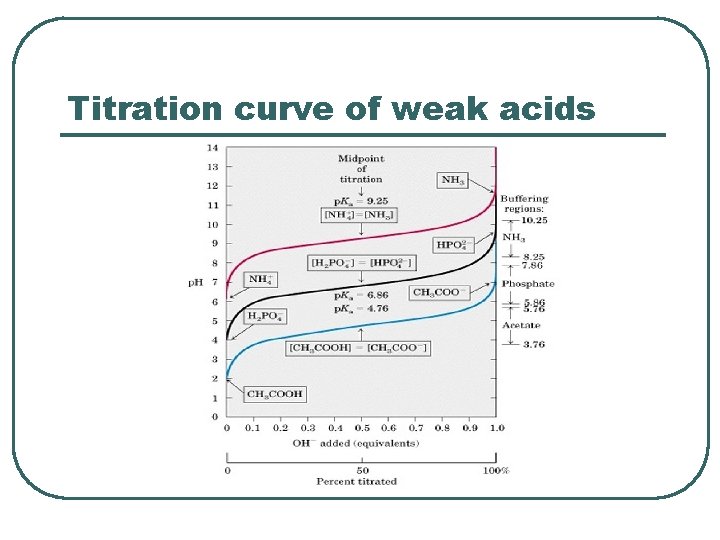
Titration curve of weak acids
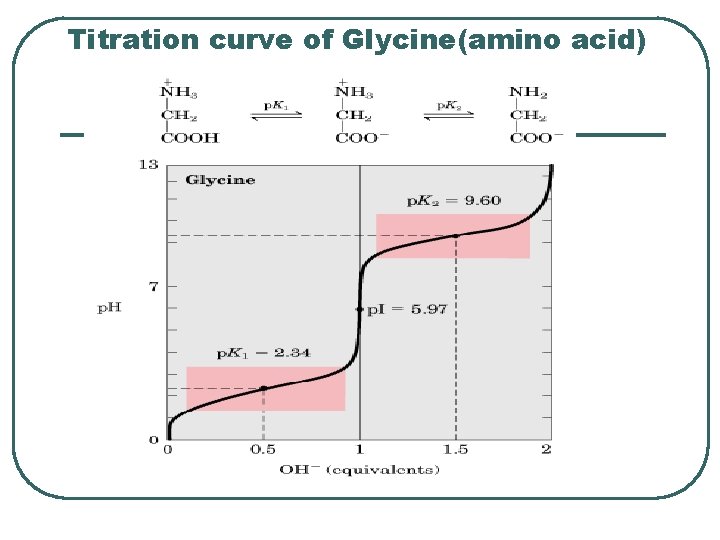
Titration curve of Glycine(amino acid)

- Slides: 44