Water Most important and abundant biological molecule Chemical
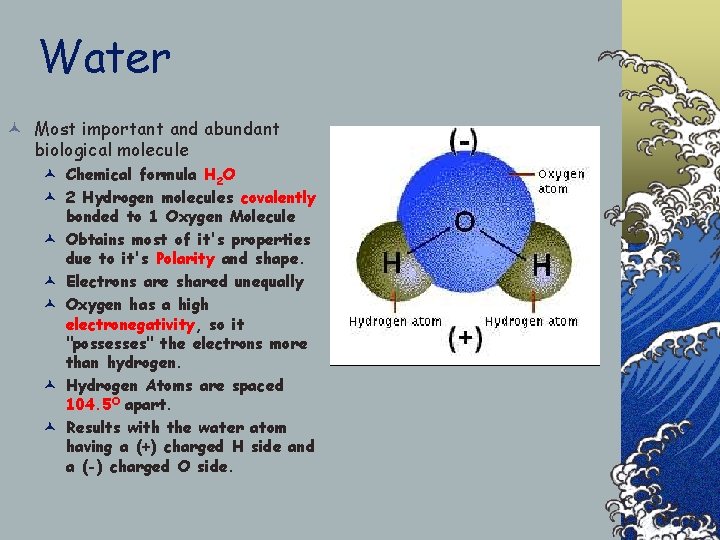
Water © Most important and abundant biological molecule © Chemical formula H 2 O © 2 Hydrogen molecules covalently bonded to 1 Oxygen Molecule © Obtains most of it's properties due to it's Polarity and shape. © Electrons are shared unequally © Oxygen has a high electronegativity, so it "possesses" the electrons more than hydrogen. © Hydrogen Atoms are spaced 104. 5 O apart. © Results with the water atom having a (+) charged H side and a (-) charged O side.
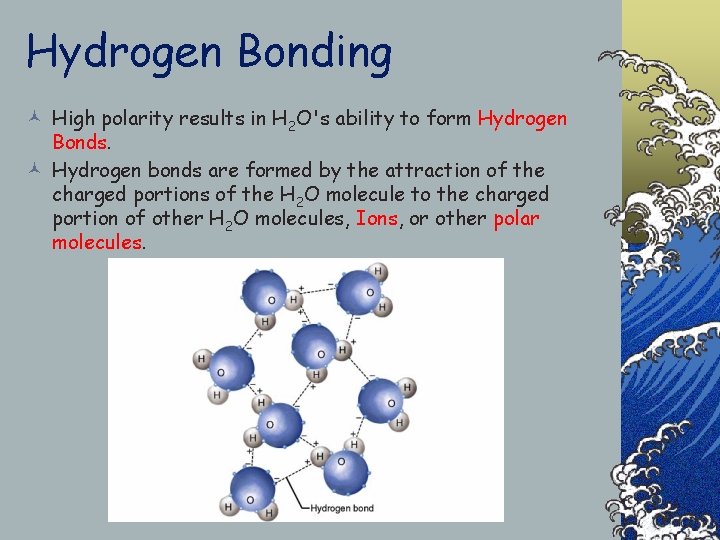
Hydrogen Bonding © High polarity results in H 2 O's ability to form Hydrogen Bonds. © Hydrogen bonds are formed by the attraction of the charged portions of the H 2 O molecule to the charged portion of other H 2 O molecules, Ions, or other polar molecules.
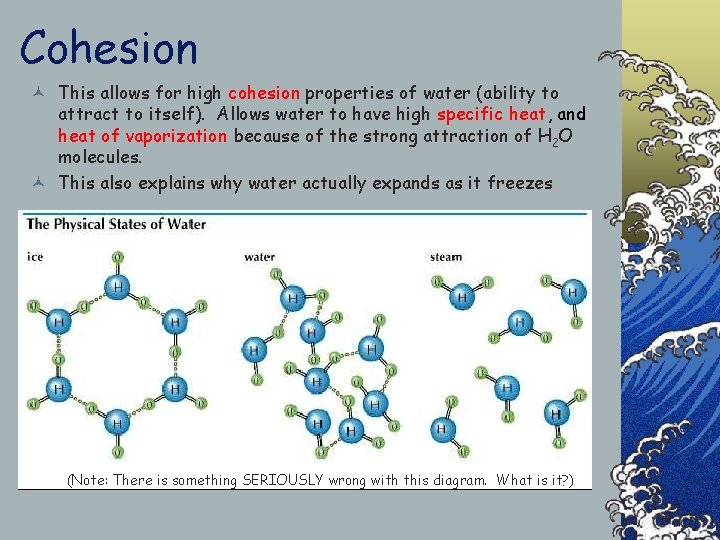
Cohesion © This allows for high cohesion properties of water (ability to attract to itself). Allows water to have high specific heat, and heat of vaporization because of the strong attraction of H 2 O molecules. © This also explains why water actually expands as it freezes (Note: There is something SERIOUSLY wrong with this diagram. What is it? )
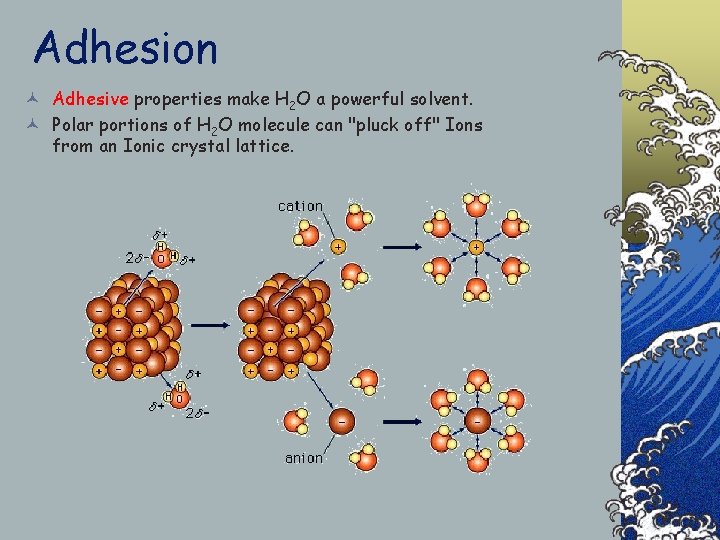
Adhesion © Adhesive properties make H 2 O a powerful solvent. © Polar portions of H 2 O molecule can "pluck off" Ions from an Ionic crystal lattice.
Interactions with Non-polar Molecules © Water Organizes nonpolar molecules. © Nonpolar molecules are hydrophobic (repelled by H 2 O) © Repulsion forces these molecules into particular arrangements. © Example is the lipid bilayer found in cellular membranes.
Water and p. H © Water disassociates (ionizes) in itself. © Basis for the p. H scale of acid - base measurement. © Water can disassociate from H 2 O to H+ (Hydrogen Ion, acid) and OH- (Hydroxide Ion, base). © Usually does this for 1/10, 000 or 1 E-7. © p. H is a measure of this dissociation. According to the following formula: p. H = -Log [H+] © Lower p. H indicates high H+ concentration - high acidity. © Higher p. H indicates low H+ concentration (high OHconcentration) - High alkalinity. © A neutral solution has p. H=7.
- Slides: 6