Water Lab Lab Activity Lab Conversation No talking

Water Lab

Lab • Activity • Lab • Conversation • No talking during directions • Normal talking during the actual lab • Help • EDs may be used to access notes or other educational material • Raise your hand if you need help • Integrity • Collaborate – do not copy answers – if your group comes to an answer and you don’t understand ask them to explain it to you! • Distribute the work fairly – don’t be a mooch. • Effort • Checking Directions • Treating lab material carefully and respectfully involve everyone in the group • Value • Hands on learning • Learning laboratory skills • Efficiency • Read the directions – most labs can be done easily by taking your time with the directions Listen

Set up Capillary Action 1. Get a paper towel 2. Fold Paper towel hotdog style 3. Place one end in cup with colored water 4. Place other end in cup with no water 5. Observe Name: Water Lab

Name: Cohesion 1. *Define Cohesion 2. Using your pipette, drop four drops of water onto your wax paper 3. *Answer: What causes the water to cling together Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together?

Name: Adhesion 1. *Define Adhesion 2. Take your water cup and one end of the string in it. 3. Put the other end of the string in the empty cup. 4. Lift up the water cup until the string is taut. 5. Carefully tip the water out of the cup and observer. 6. *Answer: What causes the water to stick to the string? Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension:
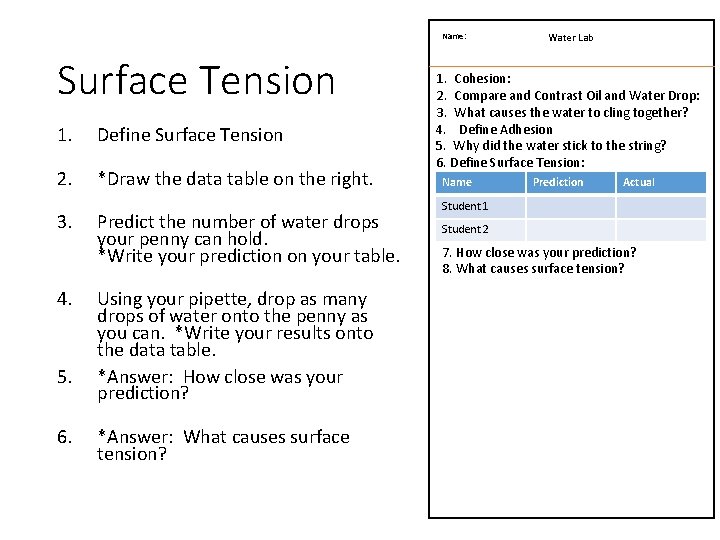
Name: Surface Tension 1. Define Surface Tension 2. *Draw the data table on the right. 3. 4. 5. 6. Predict the number of water drops your penny can hold. *Write your prediction on your table. Using your pipette, drop as many drops of water onto the penny as you can. *Write your results onto the data table. *Answer: How close was your prediction? *Answer: What causes surface tension? Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension: Name Prediction Actual Student 1 Student 2 7. How close was your prediction? 8. What causes surface tension?

Name: Surface Tension 1. Take one of your cups and fill it about ¾ of the way full 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension: Name 2. Return to your desk and carefully pour the water into your other cup until a bubble forms around the top. 3. Try to float your paper clips on the water. 4. *Answer: Why are the paper clips floating? Water Lab Prediction Actual Student 1 Student 2 7. How close was your prediction? 8. What causes surface tension? 9. Why are the paper clips floating?

Name: Universal Solvent 1. Observe the demonstration 2. Observe. 3. *Answer: What does it mean to dissolve something? 4. *Answer: Why can’t water dissolve oil? Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension: Name Prediction Actual Student 1 Student 2 7. How close was your prediction? 8. What causes surface tension? 9. Why are the paper clips floating? 10. What does it mean to dissolve something? 11. Why can’t water dissolve oil?

Name: High Specific Heat 1. Come to the front of the room to watch Ms. Roderick’s Demonstration. 2. *Answer: What happened to the cup without water? 3. *Answer: What happened to the cup with water? 4. *Answer: Why did the water cup not catch fire? Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension: Name Prediction Actual Student 1 Student 2 7. How close was your prediction? 8. What causes surface tension? 9. Why are the paper clips floating? 10. What does it mean to dissolve something? 11. Why can’t water dissolve oil? 12. What happened to the cup without water? 13. What happened to the cup with water? 14. Why did the water cup not catch fire?

Finish Capillary Action 1. Observe your capillary action experiment. Name: Water Lab 1. Cohesion: 2. Compare and Contrast Oil and Water Drop: 3. What causes the water to cling together? 4. Define Adhesion 5. Why did the water stick to the string? 6. Define Surface Tension: Name Prediction Actual Student 1 2. *Describe what has happened on your paper. 3. *Answer: How does water use adhesion and cohesion to climb up trees Student 2 7. How close was your prediction? 8. What causes surface tension? 9. Why are the paper clips floating? 10. What does it mean to dissolve something? 11. Why can’t water dissolve oil? 12. What happened to the cup without water? 13. What happened to the cup with water? 14. Why did the water cup not catch fire? 15. Describe what has happened on your paper. 16. How does water use adhesion and cohesion to climb up trees

Clean Up • Turn in your Lab • Leave your colored water cup on your table • Empty and rinse out your cups and pipettes at the sink. • Place them back on the tray. • Throw away your paper towel and wax paper. • Return Tray to your desk.
- Slides: 11