Water Impurities and Purification 1 Impurities in Water























- Slides: 40

Water Impurities and Purification 1

Impurities in Water �Suspended solids �Dissolved solids 2

Suspended Solids �These are suspensions and colloidal dispersions ◦ do not dissolve in water �The amount of suspended solids is its turbidity ◦ determined by how much light scatters as it passes through the mixture 3

Suspended Solids �Removal from water ◦ settling ◦ filtration ◦ coagulation �use aluminum sulfate Al 2(SO 4)3 4

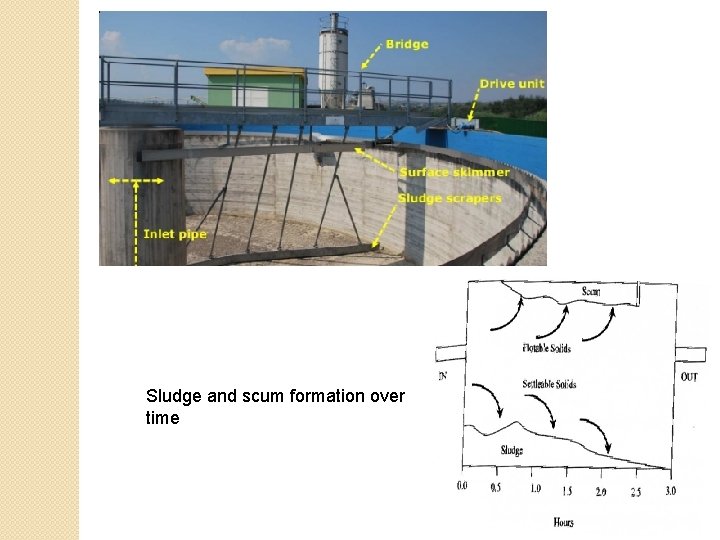
Gravity Settling Tank �Heavy suspended particles sink to the bottom of the tank ◦ removed by scrapers as sludge �Lighter matter such oils and greases float along the surface and form a scum ◦ removed by skimmers 5

Sludge and scum formation over time 6

Chemical Coagulation �Colloidal particles less that 1. 0 micron ◦ do not settle with gravity ◦ mutual repulsion due to their negative charge prevents settling �Water is treated with a coagulant such as aluminum sulfate ◦ causes particles to flocculate (clump together) �the Al 3+ ions neutralize the negative charges eliminating the repulsion ◦ these larger particles (flocs) settle out 7

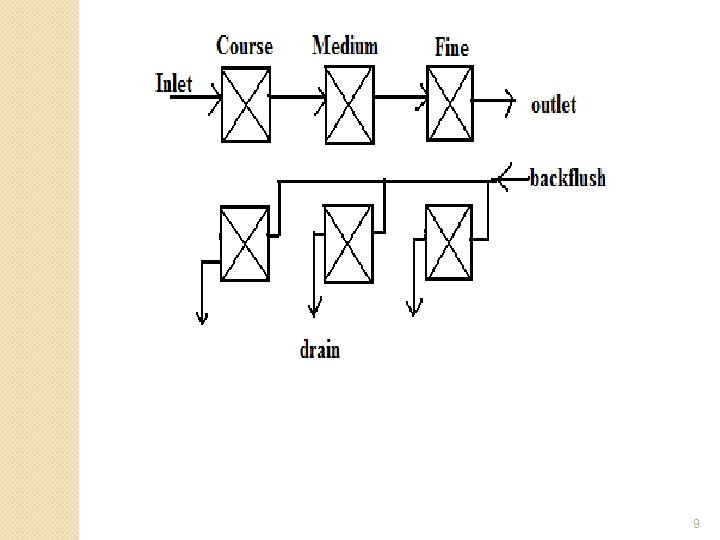
Filtration �Suspended medium. solids are trapped in the filter ◦ Filter media are �sand �gravel �carbon �Water travels from coarse to fine media �Parallel banks are set up so one bank is operational while the other is backflushed �See next slide 8

9

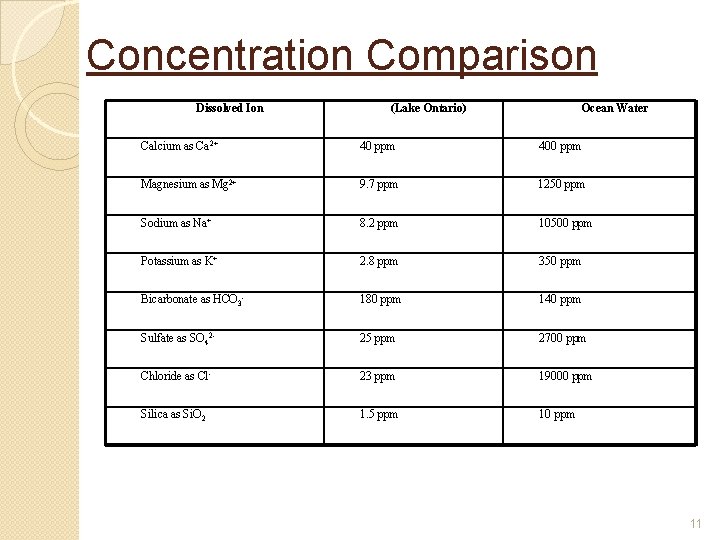
Dissolved Solids �These are ions dissolved in water as solutes �Ionic and molecular in size so too small to filter conventionally �Occur in fresh water, ground water, ocean water �Concentrations vary between fresh and ocean water 10

Concentration Comparison Dissolved Ion (Lake Ontario) Ocean Water Calcium as Ca 2+ 40 ppm 400 ppm Magnesium as Mg 2+ 9. 7 ppm 1250 ppm Sodium as Na+ 8. 2 ppm 10500 ppm Potassium as K+ 2. 8 ppm 350 ppm Bicarbonate as HCO 3 - 180 ppm 140 ppm Sulfate as SO 42 - 25 ppm 2700 ppm Chloride as Cl- 23 ppm 19000 ppm Silica as Si. O 2 1. 5 ppm 10 ppm 11

Water Hardness �Caused by dissolved solids �There are two types of water hardness: ◦ Temporary water hardness ◦ Permanent water hardness �Hard water produces scale in a boiler system 12

Temporary Water Hardness �Temporary water hardness is caused by: ◦ calcium bicarbonate, Ca(HCO 3)2 ◦ magnesium bicarbonate, Mg(HCO 3)2 ◦ temporary water hardness can be removed without chemicals �can be made to precipitate out of the water by heating the water to below boiling Ca(HCO 3)2 (aq) + heat Ca. CO 3(s)+ H 2 O + CO 2 (g) �The calcium carbonate precipitates out as sludge 13

Permanent Water Hardness �Permanent ◦ ◦ water hardness is caused by: calcium sulfate, Ca. SO 4, calcium chloride, Ca. Cl 2 magnesium sulfate, Mg. SO 4, magnesium chloride, Mg. Cl 2 �must be removed chemically �To remove these calcium and magnesium compounds the water is softened ◦ various methods are used 14

Water Softening �Water softening refers to a process used to eliminate or greatly reduce water hardness ◦ In a softening process, the calcium and magnesium compounds are reacted with sodium compounds and hydroxides �this greatly eliminates the hardness in the water and therefore the formation of scale �We will look at three types of softeners: �Lime-soda �Sodium zeolite �Demineralizers 15

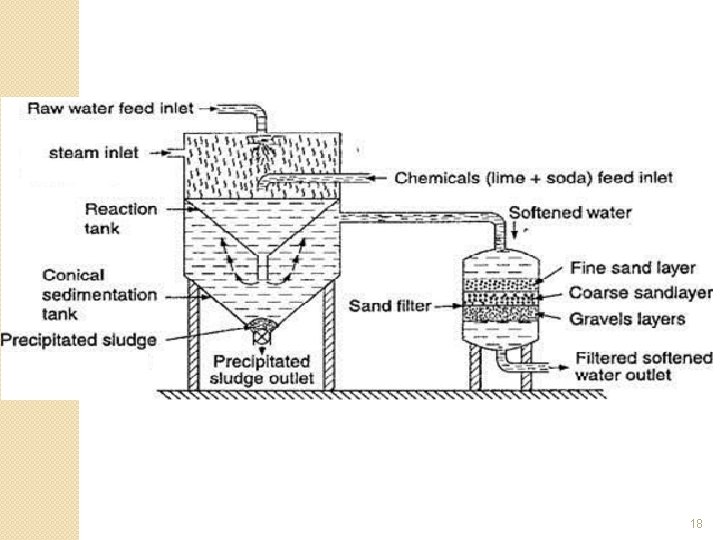
Lime-Soda Softeners �Water is heated to near boiling with steam �Ca(OH)2 (lime) and Na 2 CO 3 (soda) are reacted with calcium and magnesium salts 16

Lime-Soda Softeners �Ca. CO 3 and Mg(OH)2 settles out as sludge to be removed �The Na. Cl is highly soluble and non-scale forming �Hardness is reduced from 140 ppm to 25 ppm 17

18

Sodium Zeolite Softening �Operates on the principal of ion exchange �Zeolite is a term given to aluminosilicates which are minerals containing aluminum oxides and silicon oxides ◦ there are natural zeolites as well as many synthetic zeolites �synthetic zeolite is used for feedwater softening �a styrene/divinylbenzene resin, containing Na+ ions is used 19

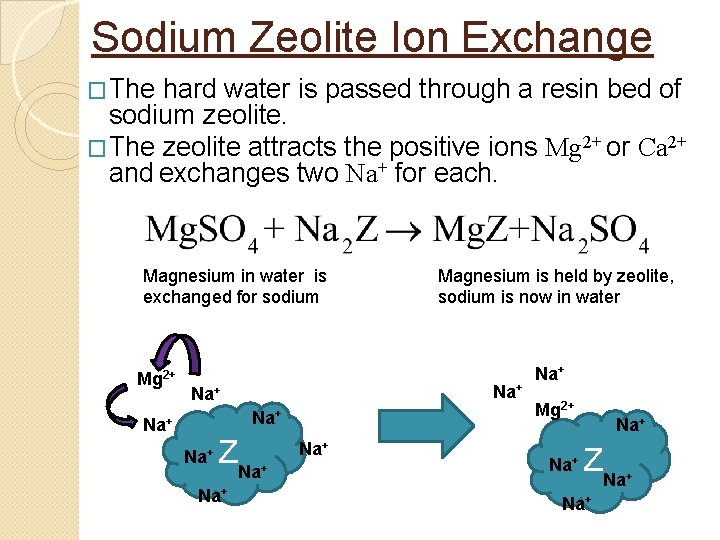
Sodium Zeolite Ion Exchange �The hard water is passed through a resin bed of sodium zeolite. �The zeolite attracts the positive ions Mg 2+ or Ca 2+ and exchanges two Na+ for each. Magnesium in water is exchanged for sodium Mg 2+ Na+ Na+ Na+ ZNa Na+ Magnesium is held by zeolite, sodium is now in water Na+ + Na+ Mg 2+ Na+ ZNa Na+ +

Sodium Zeolite Softening Products �The resulting sodium salts are highly soluble and therefore produce no scale �This process used to reduce both types of water hardness 21

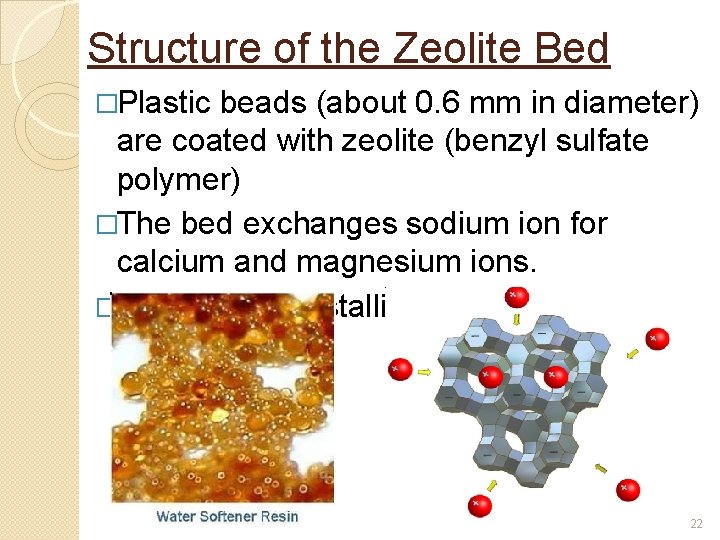
Structure of the Zeolite Bed �Plastic beads (about 0. 6 mm in diameter) are coated with zeolite (benzyl sulfate polymer) �The bed exchanges sodium ion for calcium and magnesium ions. �Zeolite is a crystalline structure 22

Regenerating the Zeolite Resin �In the zeolite softening process, the Na+ ions originally in the resin are replaced with Mg 2+ and Ca 2+ ions �A 10% Na. Cl brine is used to remove Mg 2+ and Ca 2+ ions from the resin as chloride salts and replace the two ions with Na+ 2 Na. Cl + Ca. Z Na 2 Z + Ca. Cl 2 2 Na. Cl + Mg. Z Na 2 Z + Mg. Cl 2 23

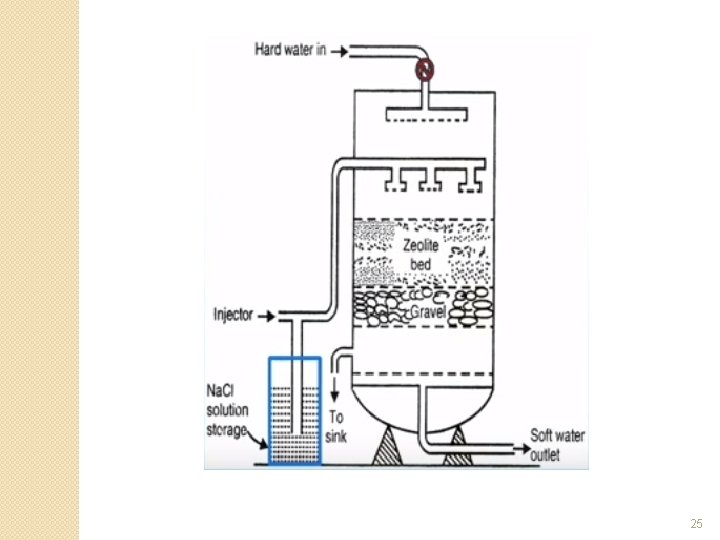
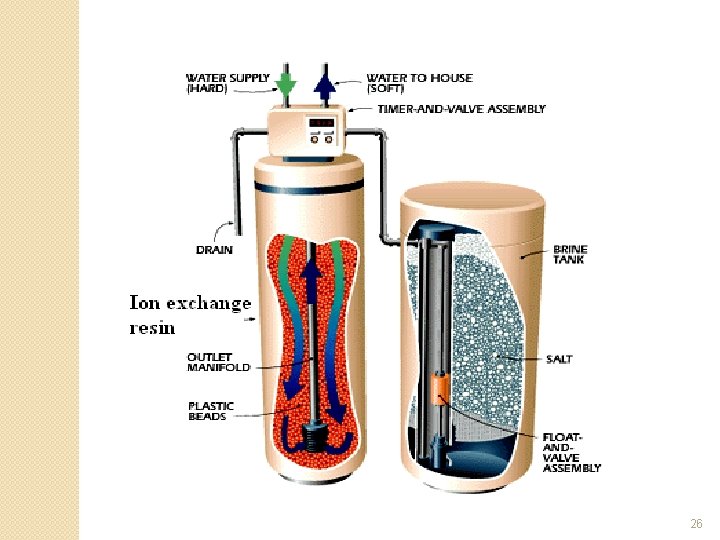
Zeolite Water Softener System �See schematic and diagram on the next two slides

25

26

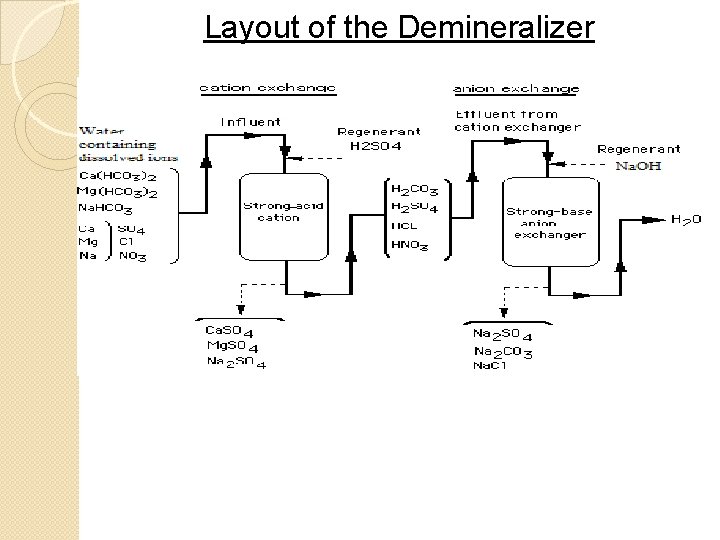
Demineralizers �Demineralizing is another ion exchange process used to soften water �Demineralizers use two zeolite based exchangers 1) An acid cation exchanger that removes all cations and replaces them with H+ ions 2) A base anion exchanger that converts the acids (from the hydrogen ion exchanger) into water and salts 27

Acid Cation Exchanger Reactions �This exchanger contains hydrogen ions that are exchanged with the cations of salts list below, producing acids ◦ Ca. SO 4, Mg. SO 4 ◦ Na. Cl ◦ Ca(HCO 3)2, Mg(HCO 3)2 �these bicarbonates produce water and CO 2 ◦ Ca. CO 3, Mg. CO 3 �these carbonates produce water and CO 2 28

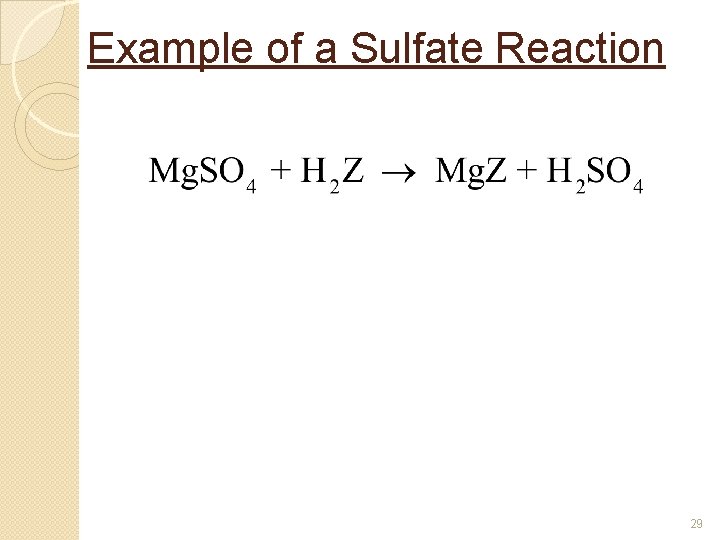
Example of a Sulfate Reaction 29

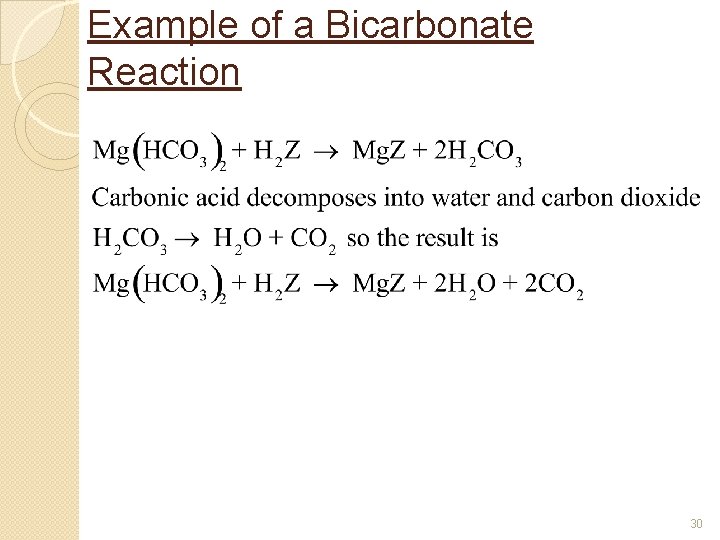
Example of a Bicarbonate Reaction 30

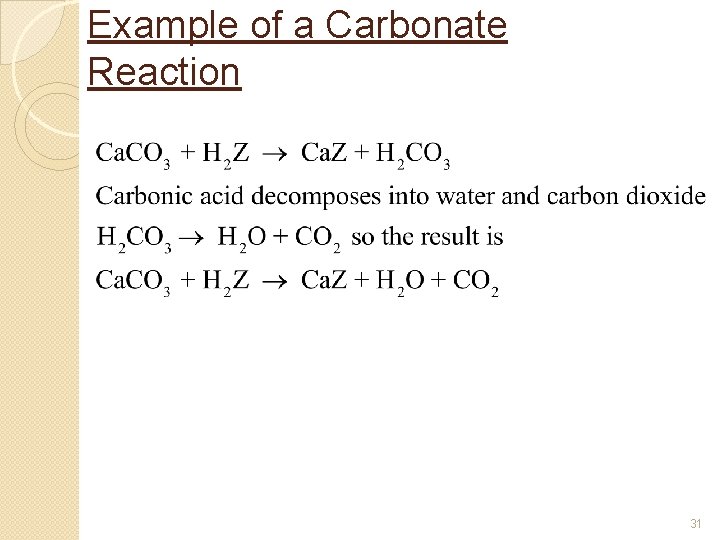
Example of a Carbonate Reaction 31

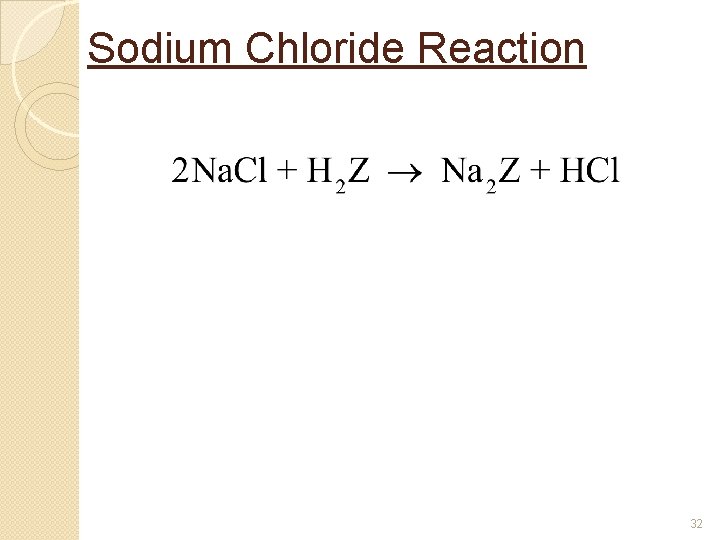
Sodium Chloride Reaction 32

Base Anion Exchanger Reactions �This second exchanger contains hydroxide ions �The zeolite base, Z(OH)2, in this second exchanger reacts with the acids produced in the first exchanger: HCl, H 2 SO 4, HNO 3, … ◦ to produce water and salts 33

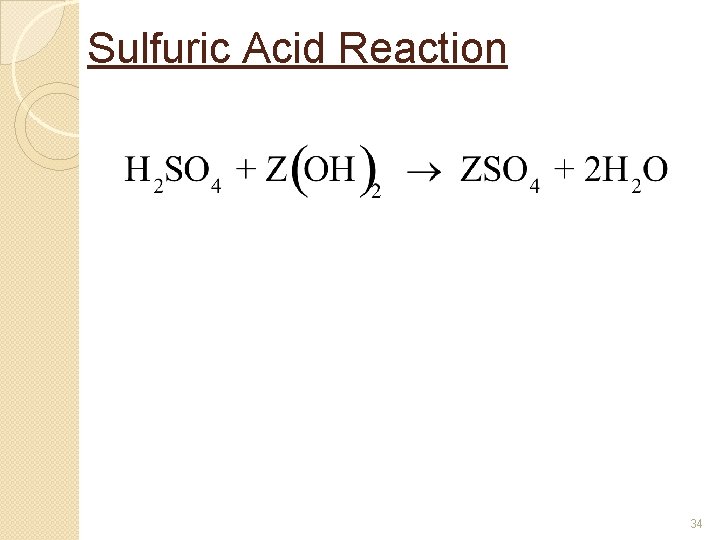
Sulfuric Acid Reaction 34

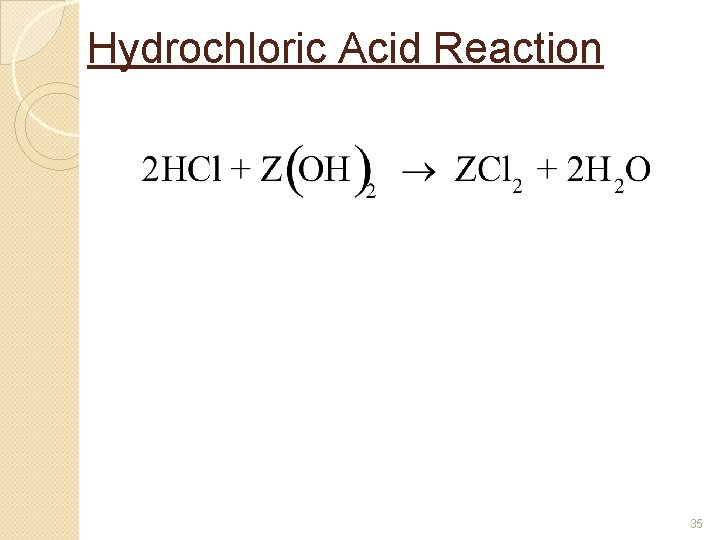
Hydrochloric Acid Reaction 35

Regenerating the Exchangers �The cation exchanger will eventually be depleted of H+ ions ◦ The resin bed is regenerated using a concentrated acid such as HCl or H 2 SO 4 �The anion exchanger will eventually be OH− ions ◦ The resin bed is regenerated using a concentrated base such as Na. OH 36

Example of Cation Exchanger Regeneration Reaction �This removes the Mg 2+ ions from the bed ◦ They form a salt that is removed from the system as waste ◦ The H+ ions are restored in the zeolite bed 37

Example of Anion Exchanger Regeneration Reaction �This removes the Cl− ions from the bed ◦ They form a salt that is removed from the system as waste ◦ The OH− ions are restored in the zeolite bed 38

39

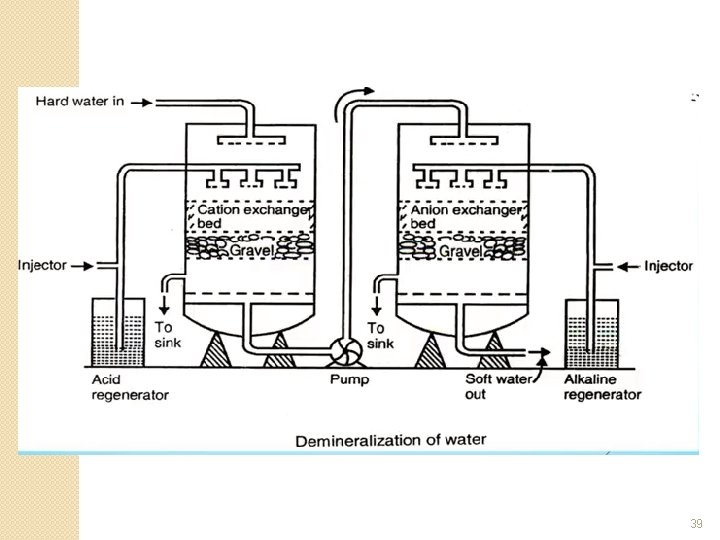
Layout of the Demineralizer