Water Filtration On the International Space Station Water









- Slides: 12

Water Filtration On the International Space Station

Water Cycle on Earth • http: //www. actewagl. com. au/education/_lib/ Flash/Water_cycle/water. swf

How we use water • In your notebooks write down as many ways that you can think of that we use water every day. 1. Wash (hands, bodies, stuff, ect) 2. Brush teeth 3. Toilet 4. Drinking

How Do We get it to Space? • We take it!!!

Water Recycling on the ISS

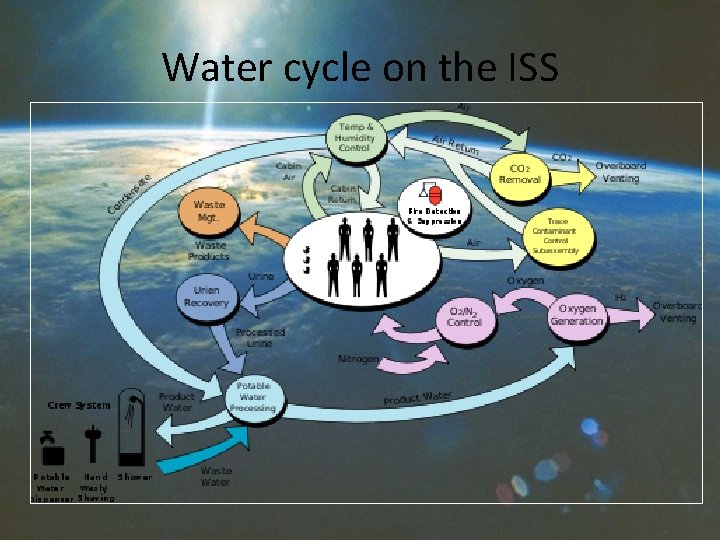
Water cycle on the ISS

ECLSS • This is a close-up view of the Environmental Control and Life Support System (ECLSS) Water Recovery System (WRS) racks. The WRS provides clean water through the reclamation of wastewaters, including water obtained from the Space Shuttle’s fuel cells, crewmember urine, used hand wash and oral hygiene water, cabin humidity condensate, and extravehicular activity (EVA) wastes

It is stored and ready use. The process isas Iodine is. The added the water to ºF control the growth of. It Then, the water istowater heated Contaminants to 265 inare a such special steam isfor condensed to form athorough. Most As chemical the contaminants flows through the removed tubes, using resins has to be. The result is pure water, a steady supply of it, and microorganisms -clean justlike chlorine isinthen added to household the reactor as oxygen is hair, skin cells, dust, etc. , relatively liquid, and the sorbents contaminants those arewhich found attracted common to joins In space, urine will undergo the most treatment. By the means to help support life in space. injected. The high temperature are filtered kills the germs the inbeen theof urine isof first sent to the urine processor water we drink atmaterials, home. Iodine isfrom used instead water these filtration devices. thereby These removing materials are packed in other types wastewater that have following a drop of urine we can see the entire where isiodine to much steam leavingto thetransport solids to water, and chemical chlorine because is easier titanium themittubes from thewastewater stream process generated –turned from such activities as contaminants composed of carbon, hydrogen, and behind orbit, and because it is less corrosive. Once all of washing, brushing teeth, housekeeping, oxygen are broken down to form carbon dioxide. these processes completed, andairthe water passes and even are humidity from the conditioning automated purity inspections system

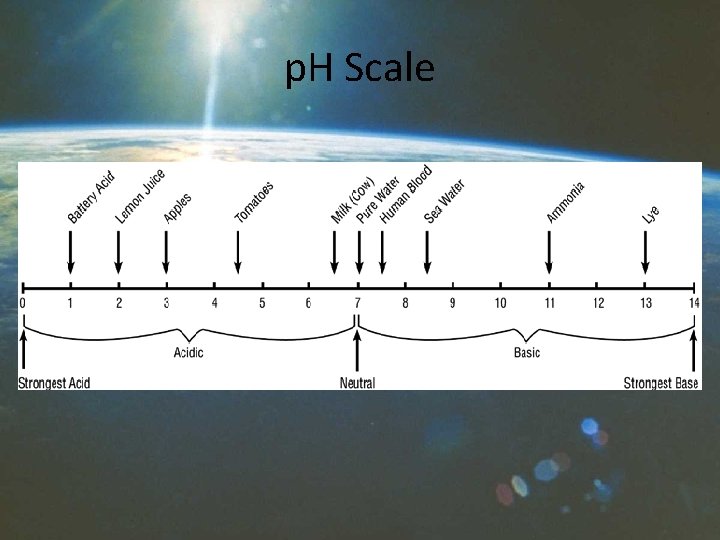
Acids And Bases » An acid is any of a class of substances that yields hydrogen ions (H+) when dissolved in water. The greater the concentration of hydrogen ions produced, the more acidic the substance is. Acids are characterized by a sour taste and the ability to react with bases and certain metals to form salts. » A base is any of a class of substances that yields hydroxide ions (OH-) when dissolved in water. The greater the concentration of hydroxide ions produced, the more basic the substance is. Bases are characterized by a bitter taste, a slippery feel, and the ability to react with acids to form salts.

p. H Scale

Challenge • Research what different materials do in filtration. • Design and draw your filtration device that will yield the purest water. • Predict what will happen. • Build your filtration system. • Test systems. • Report results.

What does it do? • Activated carbon – used to remove organic containments responsible for taste, odor, color, and clarity problems; remove chlorine and particulates • Sand – natural filter materials; acts as a strainer and traps particulates; aka mechanical or physical filtration • Cotton balls, coffee filters, cheese cloth – traps particulates; absorb water – ions, color, clarity • Macaroni & rice – absorbs; raises p. H because of Carbohydrates