Water Contamination and Human Health Water Contamination Contamination



















- Slides: 25

Water Contamination and Human Health

Water Contamination • Contamination is caused by pollution from foreign matter such as microorganisms, chemicals, industrial or other wastes, or sewage. • Many forms of water contamination can be harmful to human health. • Contamination can occur naturally, but it is mostly due to human activity.

Common Causes of Contamination • • Human and animal sewage Leakage from underground storage tanks Urban run-off Mines Landfills and waste dumps Industrial emissions and waste disposal Pesticides Agricultural run-off from crops

Types of Water Contaminants 1. 2. 3. 4. 5. 6. Microorganisms Disinfectants Disinfection byproducts Inorganic chemicals Organic chemicals Radionuclides

Example Contaminants 1. Microbial contaminants include bacteria, viruses, and parasites such as: – Cryptosporidium parasite – Giardia lambia parasite – Legionella bacteria – E. coli bacteria – Enteroviruses

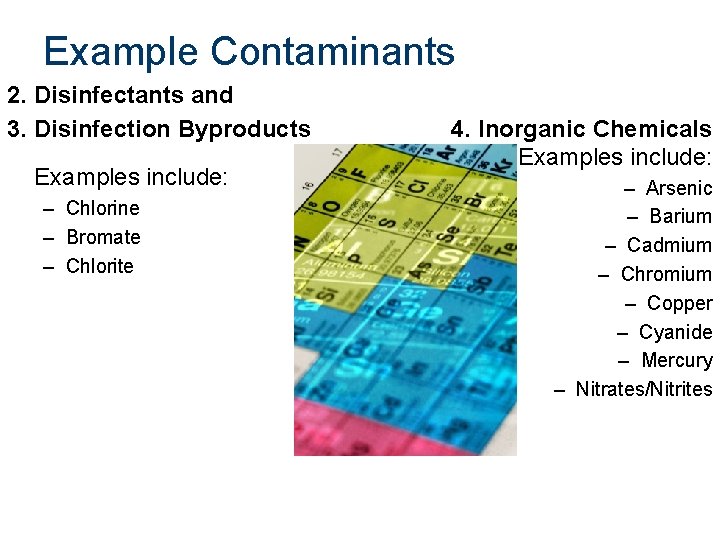
Example Contaminants 2. Disinfectants and 3. Disinfection Byproducts Examples include: – Chlorine – Bromate – Chlorite 4. Inorganic Chemicals Examples include: – Arsenic – Barium – Cadmium – Chromium – Copper – Cyanide – Mercury – Nitrates/Nitrites

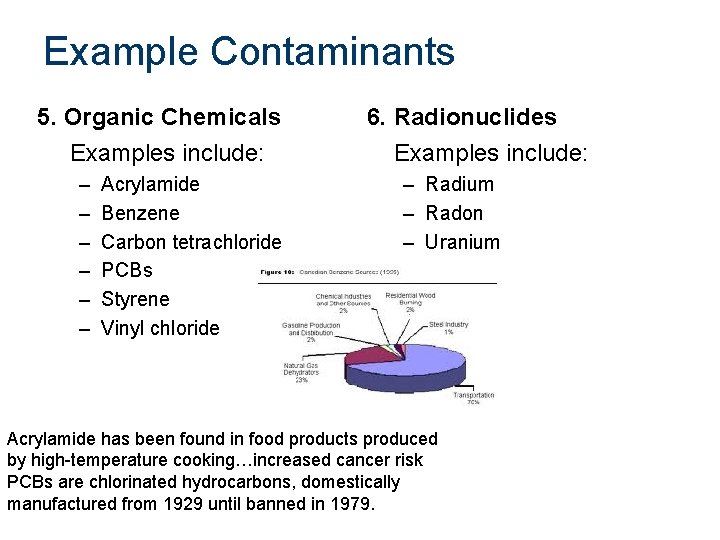
Example Contaminants 5. Organic Chemicals Examples include: – – – Acrylamide Benzene Carbon tetrachloride PCBs Styrene Vinyl chloride 6. Radionuclides Examples include: – Radium – Radon – Uranium Acrylamide has been found in food products produced by high-temperature cooking…increased cancer risk PCBs are chlorinated hydrocarbons, domestically manufactured from 1929 until banned in 1979.

Drinking Water Standards • Public water supplies are legally required to meet national standards meant to protect public health by limiting the levels of contaminants in drinking water. • Private water supplies from wells are not regulated. It is up to well owners to have their water tested.

Drinking Water Testing for: • • • Total coliforms Fecal coliforms Ammonium p. H Chlorine Chromium Copper Cyanide Iron Manganese • • • Phosphates Silica Sulfates Nitrites Radon Lead Mercury Turbidity Hardness

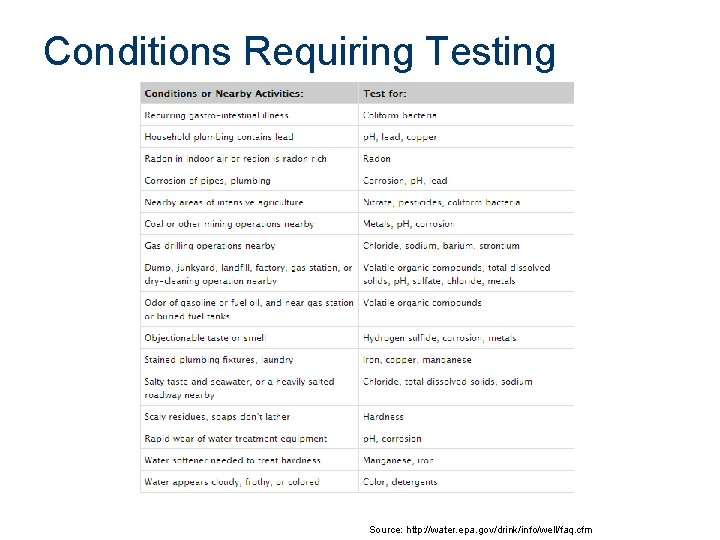
Conditions Requiring Testing Source: http: //water. epa. gov/drink/info/well/faq. cfm

Water Test You will test both the Williams’ well water as well as the water you collect from a local source for the following contaminants: • • Mercury Lead Radon Ammonium nitrogen p. H Chlorine Chromium • • Copper Cyanide Iron Nitrates Phosphates Silica Sulfide

Mercury • Mercury is a naturally occurring element found in many rocks including coal. When coal is burned, mercury is released into the environment. • Mercury in the air eventually settles into water or onto land where it can be washed into water. • Once deposited, certain microorganisms can change mercury into methylmercury, a highly toxic form that builds up in fish, shellfish, and animals that eat fish.

Lead • Lead is a naturally occurring bluish-gray metal found in small amounts in the earth's crust. • Lead can be found in all parts of our environment. Much of it comes from human activities including burning fossil fuels, mining, and manufacturing.

Radon • Radon is a cancer causing, radioactive gas. • Radon comes from the natural breakdown of uranium in soil, rock, and water and gets into the air. • Radon can get into any type of building and result in a high indoor radon level. • The greatest exposure occurs at home, where you spend most of your time. Radon is the second leading cause of lung cancer in the United States. The U. S. EPA and the Surgeon General’s office estimate radon is responsible for more than 20, 000 lung cancer deaths each year.

Ammonium • Ammonium is produced when microorganisms break down organic nitrogen products such as urea and proteins in manure. • Ammonia can lead to eutrophication, or nutrient over-enrichment, of surface waters. – The overabundance of nutrients (particularly nitrogen and phosphorus) can lead to the over-growth of algae and the resulting “blooms” can cause taste and odor problem and sometimes involve toxin-producing species.

p. H • p. H is an expression of hydrogen ion concentration in water. • p. H indicates the degree of basicity or acidity of a solution ranked on a scale of 0 to 14, with p. H 7 being neutral. – Low p. H indicates acidity and high p. H indicates basicity • Water should be neutral with a p. H close to 7. – Chemical contamination tends to make water acidic or basic

Chlorine • The gaseous or liquid form of chlorine (Cl 2) is a water additive used by municipal water systems to control microbes. • Some people who use water containing chlorine products in excess of the maximum residual disinfectant level could experience irritating effects to their eyes and nose, stomach discomfort, nervous system effects, or anemia.

Chromium • Chromium, a metallic element, is found in rocks, soil, plants, and animals. It is also used in steel making, metal plating, leather tanning, paints, dyes, and wood preservatives. • Chromium-3 has relatively low toxicity and would be a concern in drinking water only at very high levels of contamination, unlike chromium-6 and -0, which are more toxic and pose potential health risks to people.

Copper • Copper is a metal found in natural deposits such as ores containing other elements. • Copper is widely used in household plumbing materials. • Copper may cause health problems if present in public or private water supplies in amounts greater than the drinking water standard set by EPA.

Cyanide • Cyanide is a carbon-nitrogen chemical unit. • The most commonly used form, hydrogen cyanide, is mainly used to make compounds and other synthetic fibers and resins. • Cyanide may cause health problems if present in public or private water supplies in amounts greater than the drinking water standard set by EPA.

Iron • Iron is a trace element needed by plants and animals in small amounts. • Iron is derived from minerals in the soil and underlying rocks. • Presence of iron in water results in “hard” water and creates an unpleasant odor and taste. • Iron is not considered to present a risk to human health.

Nitrates • Nitrates and nitrites are nitrogen-oxygen chemical units which combine with various organic and inorganic compounds. • The greatest use of nitrates is as a fertilizer. Once taken into the body, nitrates are converted to nitrites. • Nitrates may cause health problems if present in amounts greater than the drinking water standard set by EPA.

Phosphates • Phosphorus is one of the key elements necessary for growth of plants and animals. • Phosphates in high amounts are often due to use of fertilizers and organic pesticides. • Phosphates do not affect human health unless present in very high amounts.

Silica • Silica, the chemical compound silicon dioxide, is most commonly found in nature as sand or quartz • Compelling data suggest that silica is essential for healthough no RDI has been established. Little toxicity data exist regarding aqueous silica consumption due, in part, to the lack of anecdotal reports of toxicity and general presumption of safety.

Sulfide • Sulfates are one form of sulfur species found in water. • Pesticide residuals and manufacturing wastes are sources of pollutant sulfur species. • Sulfates are reduced by a strain of bacteria to hydrogen sulfide, which give the water a rotten egg smell. • Sulfates in large amounts may cause negative health effects.