Water Composition of the Human Body Water Makes
Water
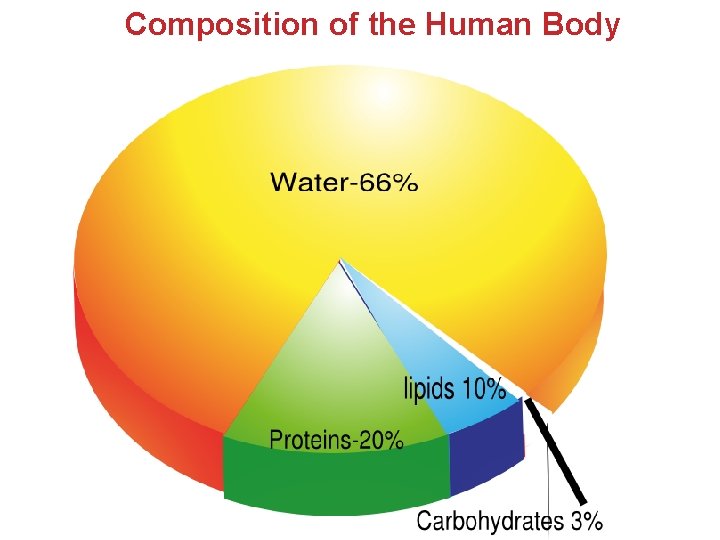
Composition of the Human Body
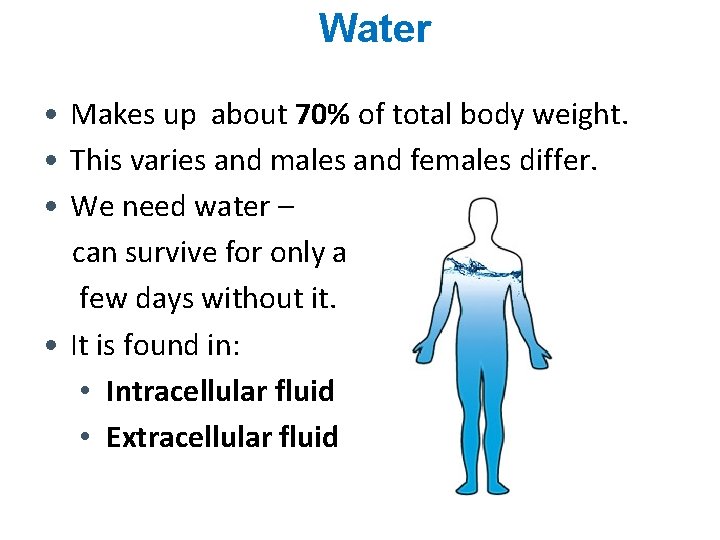
Water • Makes up about 70% of total body weight. • This varies and males and females differ. • We need water – can survive for only a few days without it. • It is found in: • Intracellular fluid • Extracellular fluid
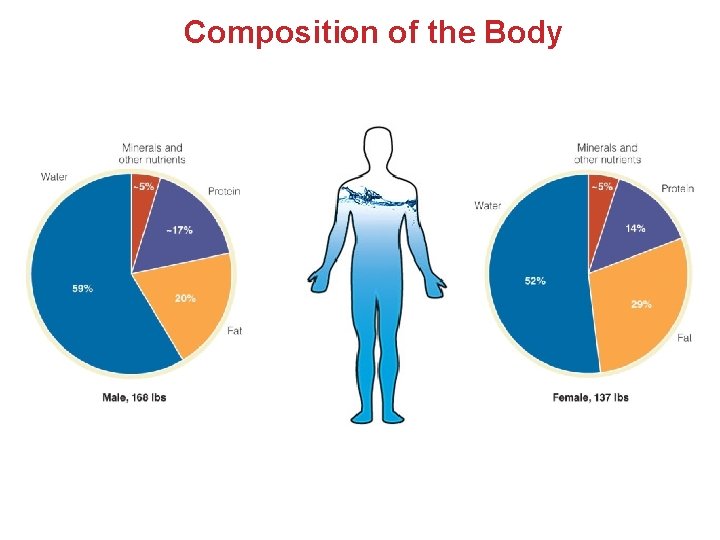
Composition of the Body
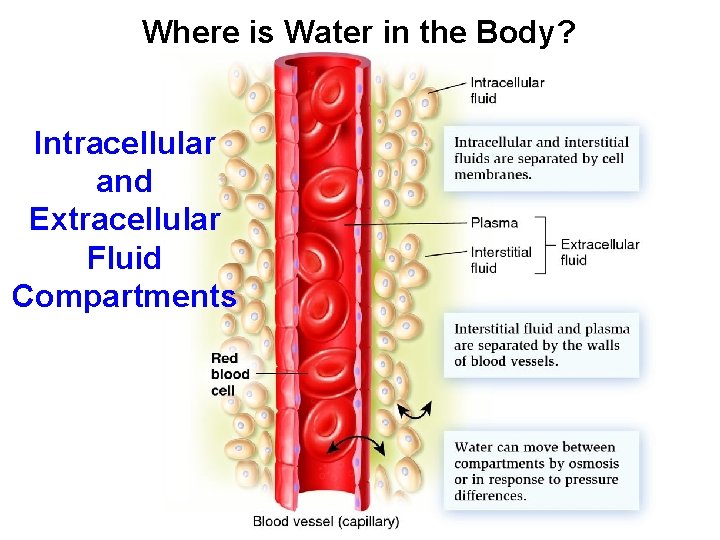
Where is Water in the Body? Intracellular and Extracellular Fluid Compartments
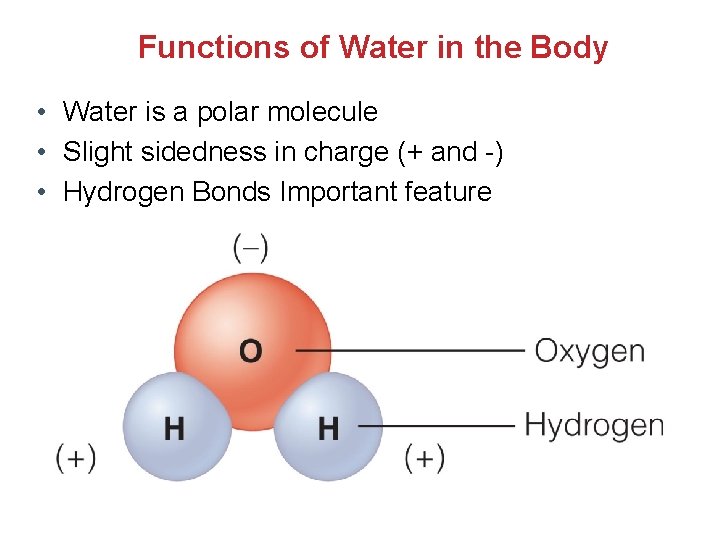
Functions of Water in the Body • Water is a polar molecule • Slight sidedness in charge (+ and -) • Hydrogen Bonds Important feature
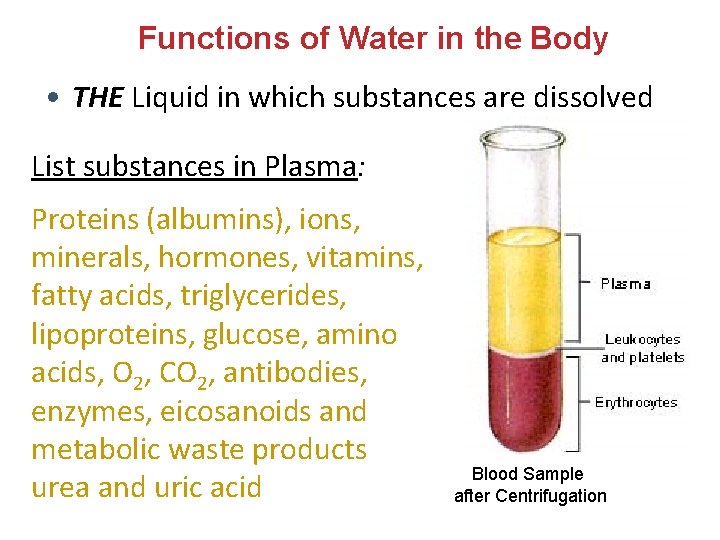
Functions of Water in the Body • THE Liquid in which substances are dissolved List substances in Plasma: Proteins (albumins), ions, minerals, hormones, vitamins, fatty acids, triglycerides, lipoproteins, glucose, amino acids, O 2, CO 2, antibodies, enzymes, eicosanoids and metabolic waste products urea and uric acid Blood Sample after Centrifugation
Functions of Water in the Body • Transport Nutrients throughout the body • Transports O 2 and hormones to cells • Removes Waste products for Excretion in Urine and Feces • Important to Digestion – required for the breakdown (Hydrolysis) of the 3 organic macronutrients that are energy yielding. plasma

Functions of Water in the Body • Maintains Tb (Body Temperature) • Especially Regulation of Cooling! • Important Lubricant for: • Joints • Eyes • Mouth and Gastrointestinal Tract • Lungs • Urogenital Tract Tears and saliva are ~ 95% water, but also contain oils, proteins and antimicrobial agents (like lysozymes). Also in human milk and mucus.
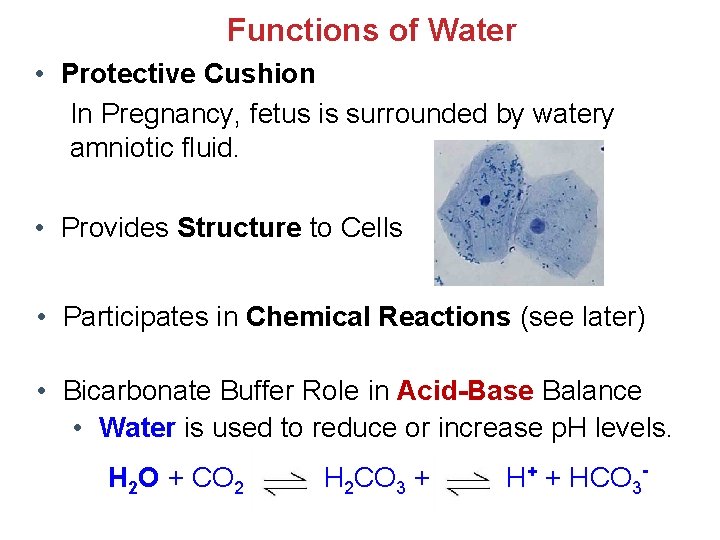
Functions of Water • Protective Cushion In Pregnancy, fetus is surrounded by watery amniotic fluid. • Provides Structure to Cells • Participates in Chemical Reactions (see later) • Bicarbonate Buffer Role in Acid-Base Balance • Water is used to reduce or increase p. H levels. H 2 O + CO 2 H 2 CO 3 + H+ + HCO 3 -
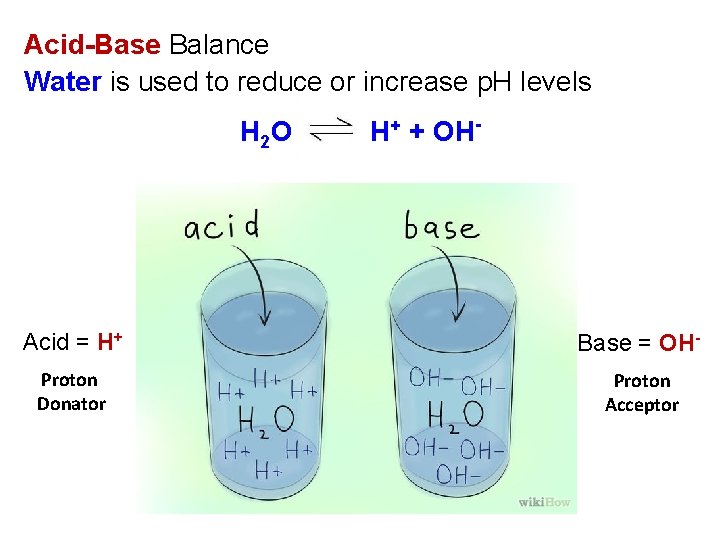
Acid-Base Balance Water is used to reduce or increase p. H levels H 2 O H+ + OH- Acid = H+ Base = OH- Proton Donator Proton Acceptor
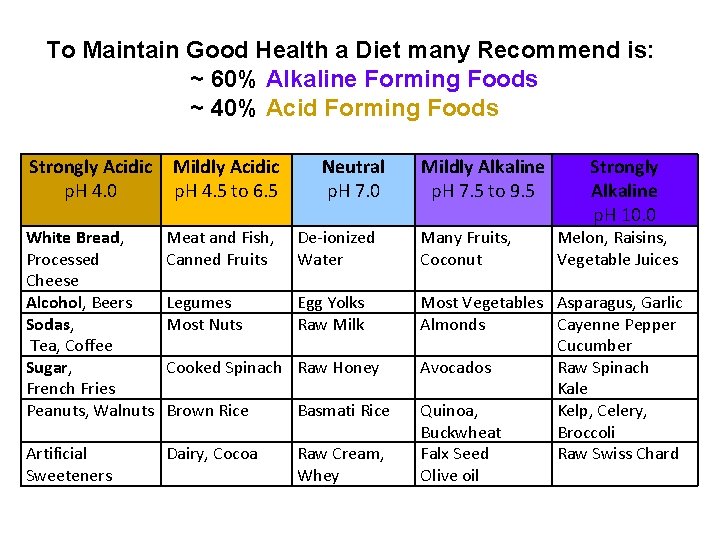
To Maintain Good Health a Diet many Recommend is: ~ 60% Alkaline Forming Foods ~ 40% Acid Forming Foods Strongly Acidic p. H 4. 0 Mildly Acidic p. H 4. 5 to 6. 5 Neutral p. H 7. 0 White Bread, Processed Cheese Alcohol, Beers Sodas, Tea, Coffee Sugar, French Fries Peanuts, Walnuts Meat and Fish, Canned Fruits De-ionized Water Many Fruits, Coconut Legumes Most Nuts Egg Yolks Raw Milk Brown Rice Basmati Rice Artificial Sweeteners Dairy, Cocoa Raw Cream, Whey Most Vegetables Asparagus, Garlic Almonds Cayenne Pepper Cucumber Avocados Raw Spinach Kale Quinoa, Kelp, Celery, Buckwheat Broccoli Falx Seed Raw Swiss Chard Olive oil Cooked Spinach Raw Honey Mildly Alkaline p. H 7. 5 to 9. 5 Strongly Alkaline p. H 10. 0 Melon, Raisins, Vegetable Juices

What are the 4 Properties of Water? 1) Solvency 2) Cohesion 3) Thermostability 4) Reactivity
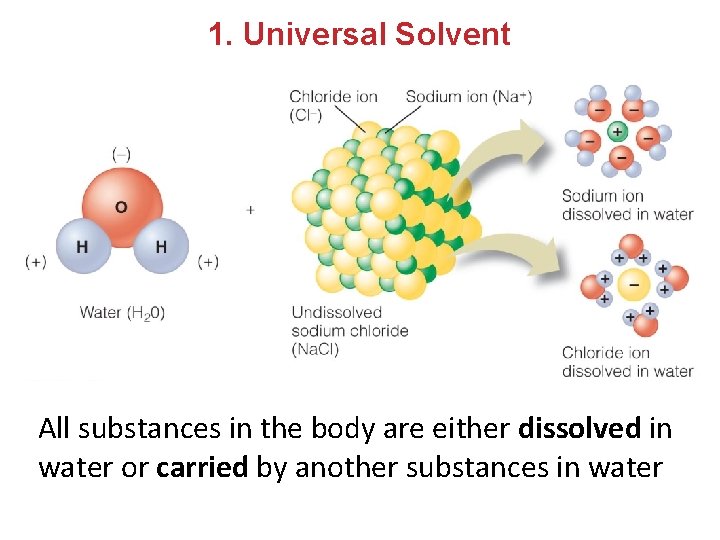
1. Universal Solvent All substances in the body are either dissolved in water or carried by another substances in water
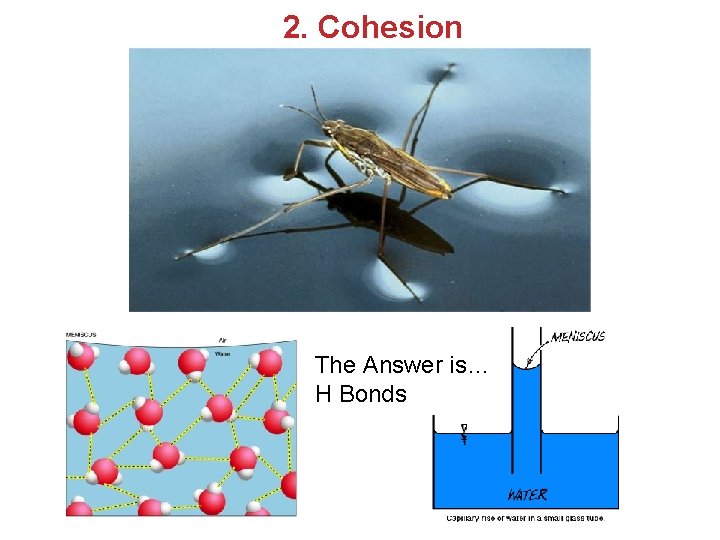
2. Cohesion The Answer is… H Bonds
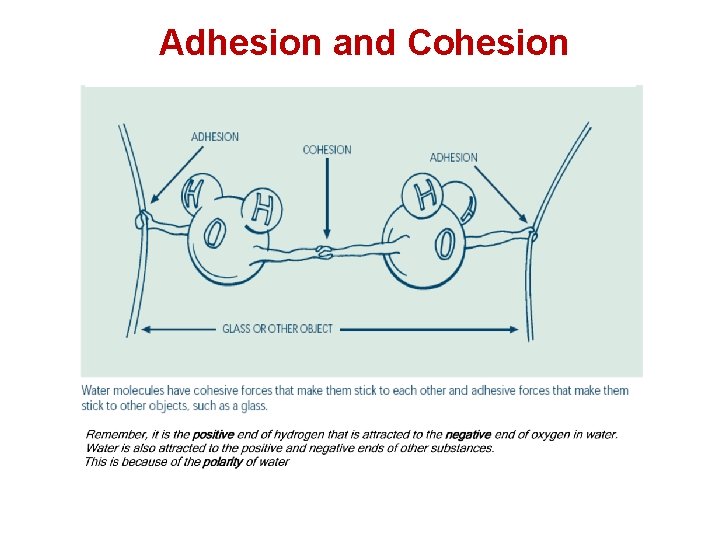
Adhesion and Cohesion
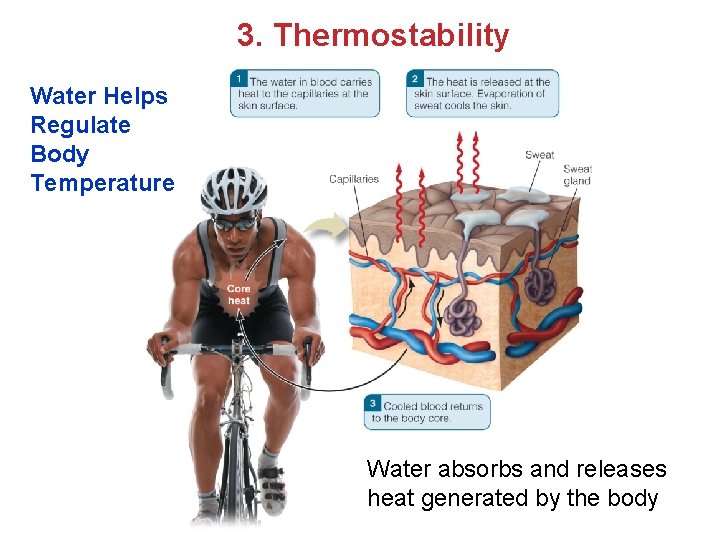
3. Thermostability Water Helps Regulate Body Temperature Water absorbs and releases heat generated by the body
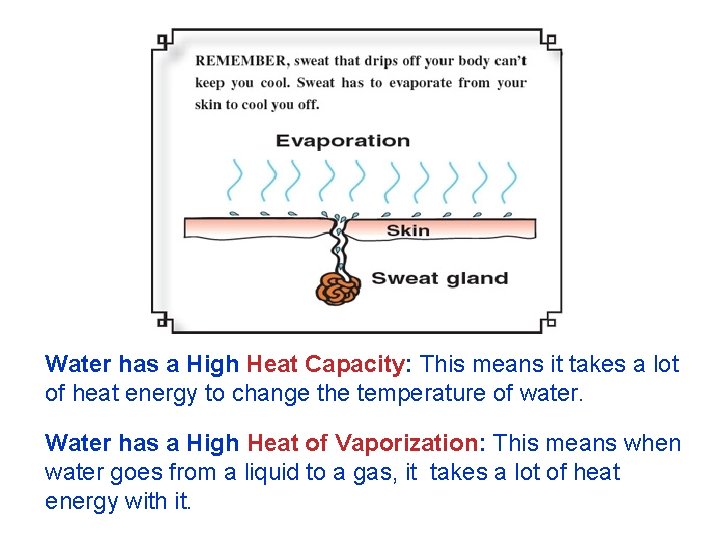
Water has a High Heat Capacity: This means it takes a lot of heat energy to change the temperature of water. Water has a High Heat of Vaporization: This means when water goes from a liquid to a gas, it takes a lot of heat energy with it.
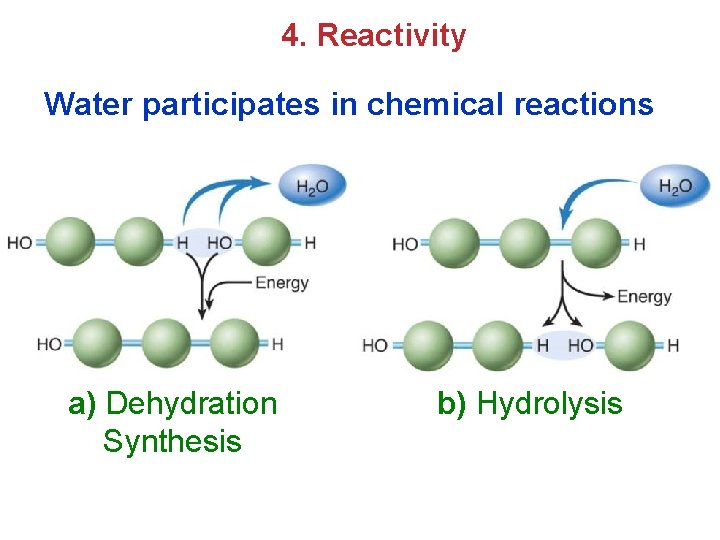
4. Reactivity Water participates in chemical reactions a) Dehydration Synthesis b) Hydrolysis
How to Maintained Balance?

How do we Maintained Water Balance? Fluid Balance is a crucial part of Homeostasis “Constantly changing to stay the same” Water Balance Water Consumed = Water Excreted Our Bodies must Adapt Quickly and Effectively to Changes in Water Intake and Water Losses Key Factors: Water Intake = Diet Water Loss = Kidneys

Sources of Body Water in Diet! • Beverages are the largest source of Water. • All foods contain some water. • Fruits and Vegetables contain the most. • Grains contain the least. • Metabolic H 2 O (~300 ml/day) • Water generated during metabolism C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + E • These sources contribute to an average daily intake of 2, 550 ml (about 2 quarts).
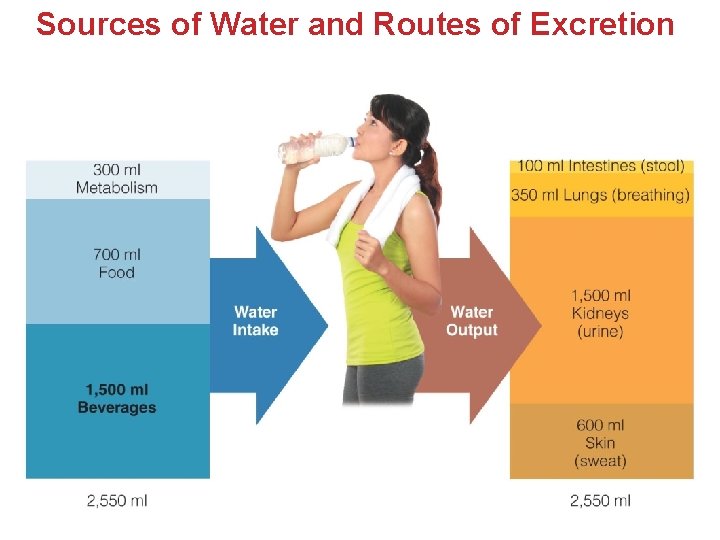
Sources of Water and Routes of Excretion

Water Excreted via kidneys, Large Intestine, Lungs, and Skin • Majority of fluid is excreted via Kidneys as Urine • Urine production depends water intake. (1. 5 L) • Water is lost through Large Intestine in the stool. • Level of Fibers can effect excretion. (100 ml) • Diarrhea and vomiting can increase excretion. • Insensible Water Loss • Evaporated from Lungs via Exhalation (350 ml) • From Perspiration of the Skin (600 ml)

Sweat The degree of Water Loss through Sweat Varies: Environmental factors – Temperature – Humidity – Wind – Sun's intensity – Clothing worn – Amount of physical activity Sweat also Contains: Lactate, lysozyme, urea, minerals (sodium, chloride, potassium, calcium, magnesium + trace elements) exogenous organic compounds; p. H ranges from 4. 5 and 7. 0.
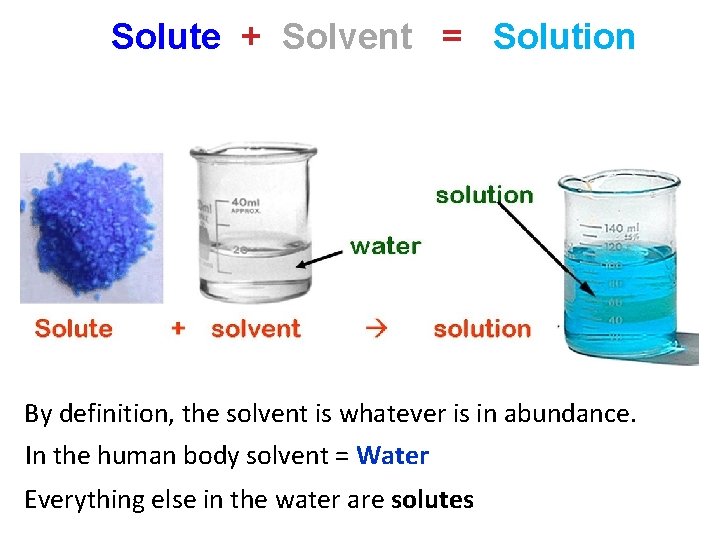
Solute + Solvent = Solution By definition, the solvent is whatever is in abundance. In the human body solvent = Water Everything else in the water are solutes
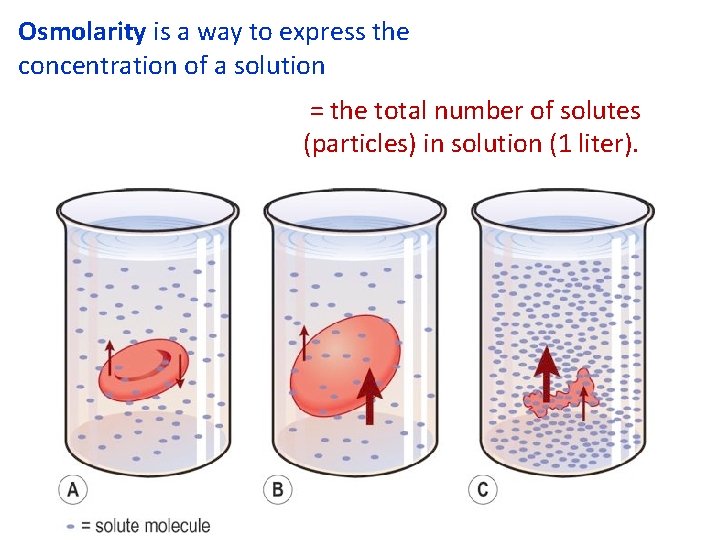
Osmolarity is a way to express the concentration of a solution = the total number of solutes (particles) in solution (1 liter).
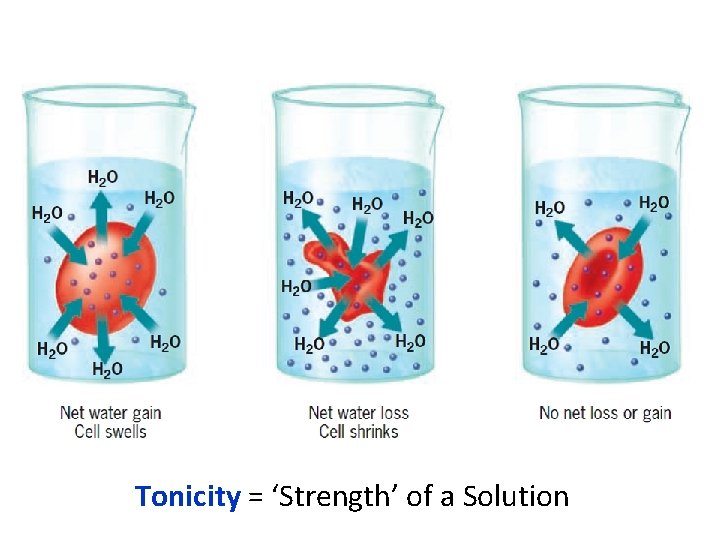
Tonicity = ‘Strength’ of a Solution
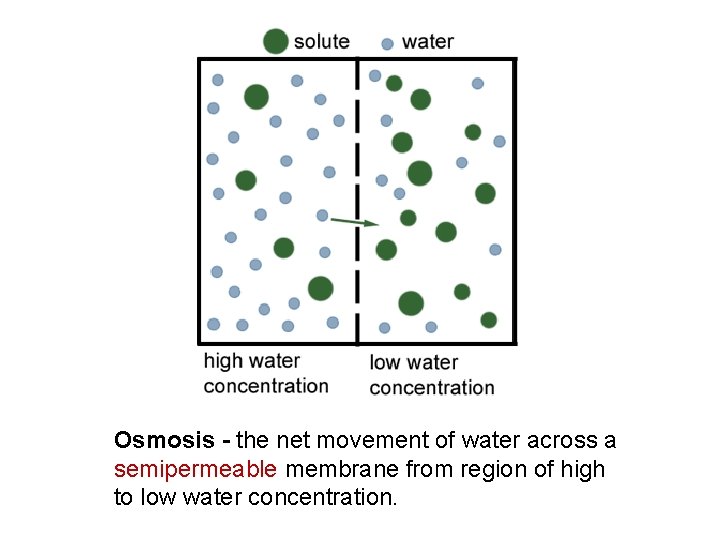
Osmosis - the net movement of water across a semipermeable membrane from region of high to low water concentration.
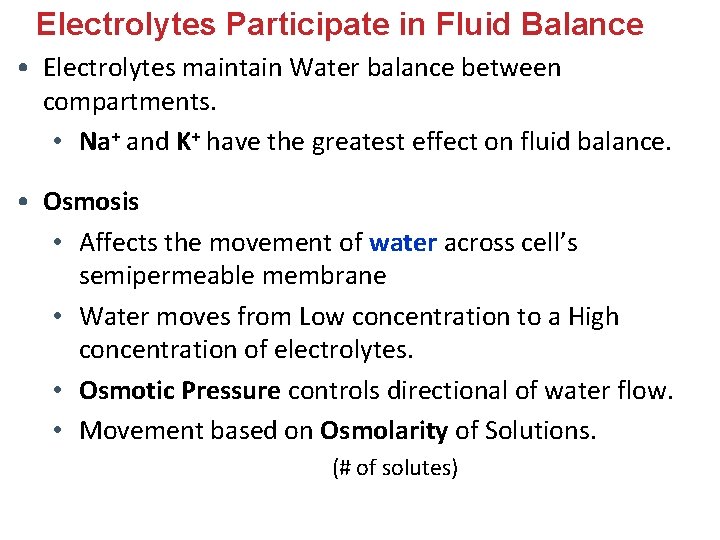
Electrolytes Participate in Fluid Balance • Electrolytes maintain Water balance between compartments. • Na+ and K+ have the greatest effect on fluid balance. • Osmosis • Affects the movement of water across cell’s semipermeable membrane • Water moves from Low concentration to a High concentration of electrolytes. • Osmotic Pressure controls directional of water flow. • Movement based on Osmolarity of Solutions. (# of solutes)

Water moves across cell membranes by osmosis
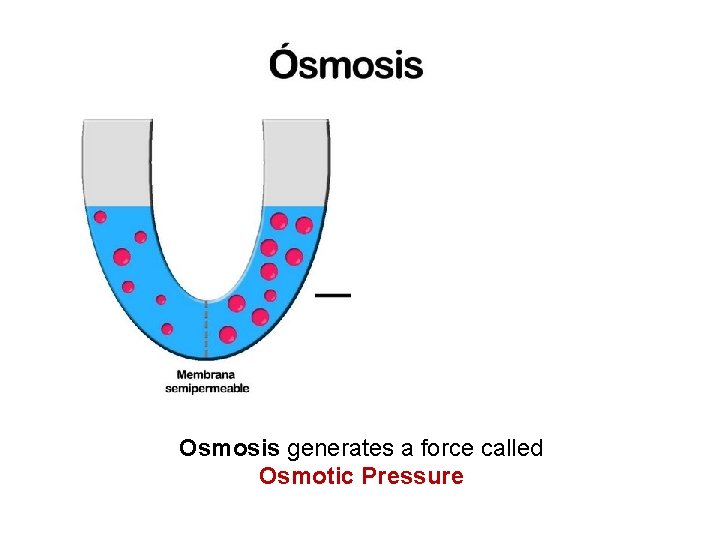
Osmosis generates a force called Osmotic Pressure
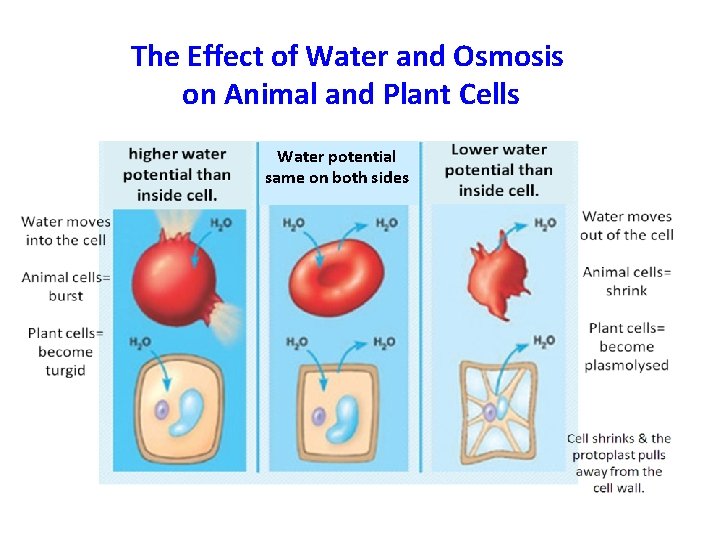
The Effect of Water and Osmosis on Animal and Plant Cells Water potential same on both sides
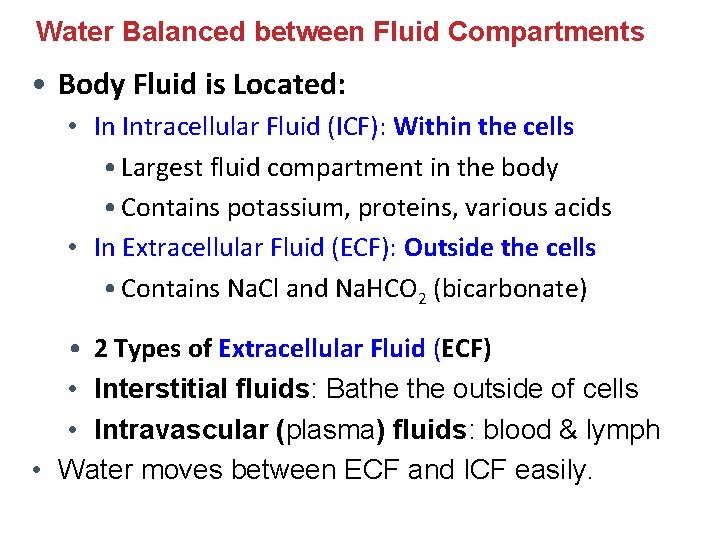
Water Balanced between Fluid Compartments • Body Fluid is Located: • In Intracellular Fluid (ICF): Within the cells • Largest fluid compartment in the body • Contains potassium, proteins, various acids • In Extracellular Fluid (ECF): Outside the cells • Contains Na. Cl and Na. HCO 2 (bicarbonate) • 2 Types of Extracellular Fluid (ECF) • Interstitial fluids: Bathe outside of cells • Intravascular (plasma) fluids: blood & lymph • Water moves between ECF and ICF easily.

Intracellular and Extracellular Fluid Compartments
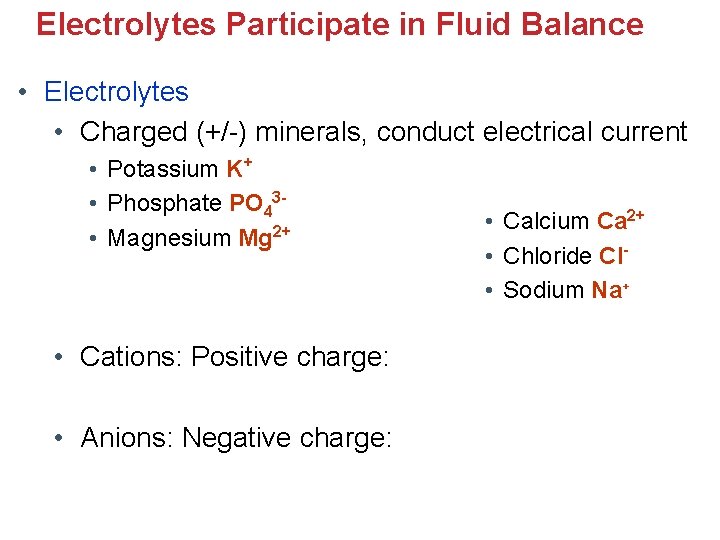
Electrolytes Participate in Fluid Balance • Electrolytes • Charged (+/-) minerals, conduct electrical current • Potassium K+ • Phosphate PO 43 • Magnesium Mg 2+ • Cations: Positive charge: • Anions: Negative charge: • Calcium Ca 2+ • Chloride Cl • Sodium Na+
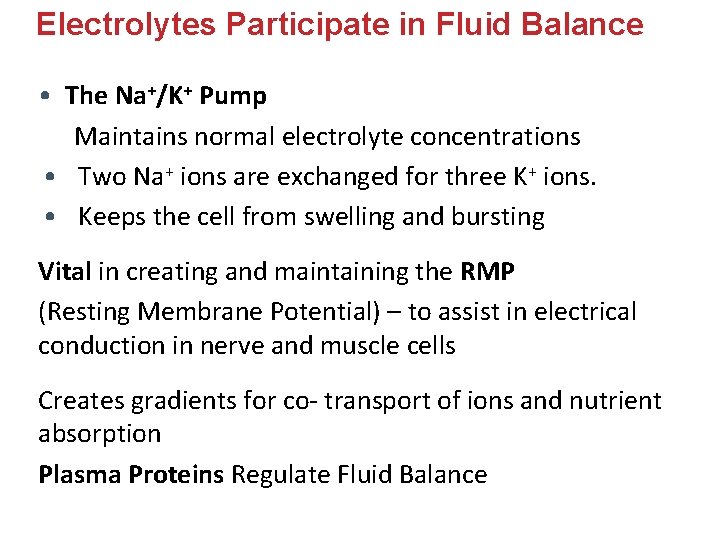
Electrolytes Participate in Fluid Balance • The Na+/K+ Pump Maintains normal electrolyte concentrations • Two Na+ ions are exchanged for three K+ ions. • Keeps the cell from swelling and bursting Vital in creating and maintaining the RMP (Resting Membrane Potential) – to assist in electrical conduction in nerve and muscle cells Creates gradients for co- transport of ions and nutrient absorption Plasma Proteins Regulate Fluid Balance
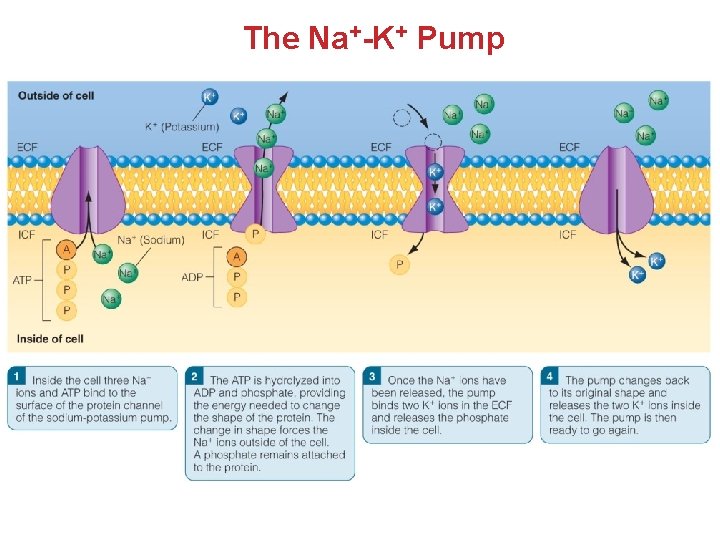
The Na+-K+ Pump
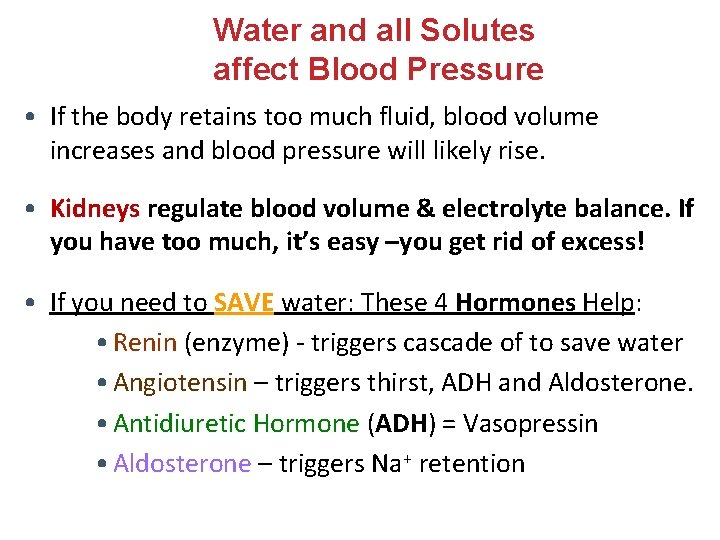
Water and all Solutes affect Blood Pressure • If the body retains too much fluid, blood volume increases and blood pressure will likely rise. • Kidneys regulate blood volume & electrolyte balance. If you have too much, it’s easy –you get rid of excess! • If you need to SAVE water: These 4 Hormones Help: • Renin (enzyme) - triggers cascade of to save water • Angiotensin – triggers thirst, ADH and Aldosterone. • Antidiuretic Hormone (ADH) = Vasopressin • Aldosterone – triggers Na+ retention

Renin Helps Conserve Water and Salts • Blood Pressure falls or Na+ concentration is reduced. • Renin is secreted by the kidneys. • Enzyme activates angiotensin I from the inert protein angiotensinogen in the blood. • In the lungs, angiotensin I is converted to Angiotensin II – this then does the following: q Triggers Thirst (in hypothalamus) q Stimulates release of Antidiuretic Hormone (ADH) q Stimulates adrenal glands to release Aldosterone q Is a powerful vasoconstrictor
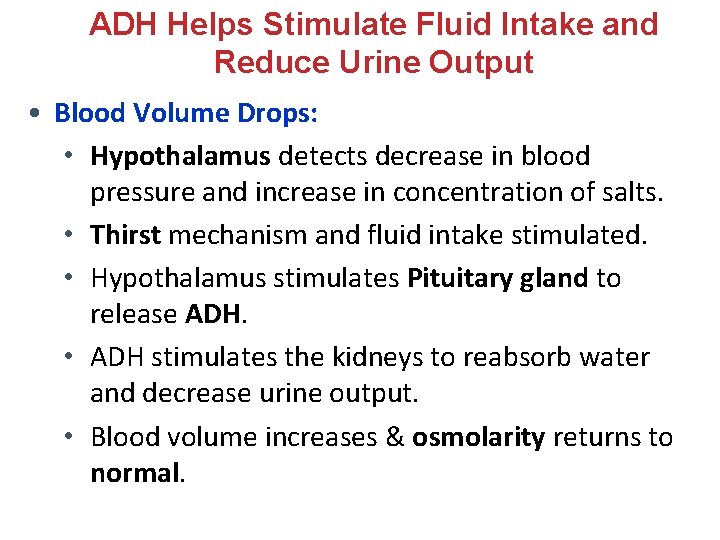
ADH Helps Stimulate Fluid Intake and Reduce Urine Output • Blood Volume Drops: • Hypothalamus detects decrease in blood pressure and increase in concentration of salts. • Thirst mechanism and fluid intake stimulated. • Hypothalamus stimulates Pituitary gland to release ADH. • ADH stimulates the kidneys to reabsorb water and decrease urine output. • Blood volume increases & osmolarity returns to normal.

Aldosterone and Sodium Reabsorption • Renin-Angiotensin system adapts to Hydration. • Too little sodium • Osmolarity drops in extracellular fluid (ECF). • Fluid shifts from blood to interstitial fluid. • Blood volume and blood pressure decrease. • Angiotensin II triggers the adrenal glands to release aldosterone. • Aldosterone • Signals kidneys to retain more Na+ • Indirectly leads to water retention
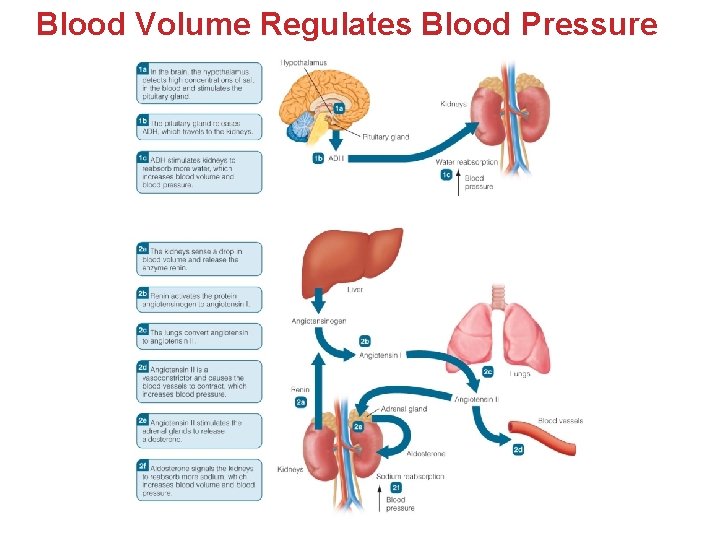
Blood Volume Regulates Blood Pressure

How Much Water Do You Need? • Water Needs Depend on: • Physical Activity • Environmental Factors • Diet Note: About 80% intake from beverages and 20% from food (small % metabolic). Keep Hydrated! Use your observational skills to determine if you are over or de-hydrated.
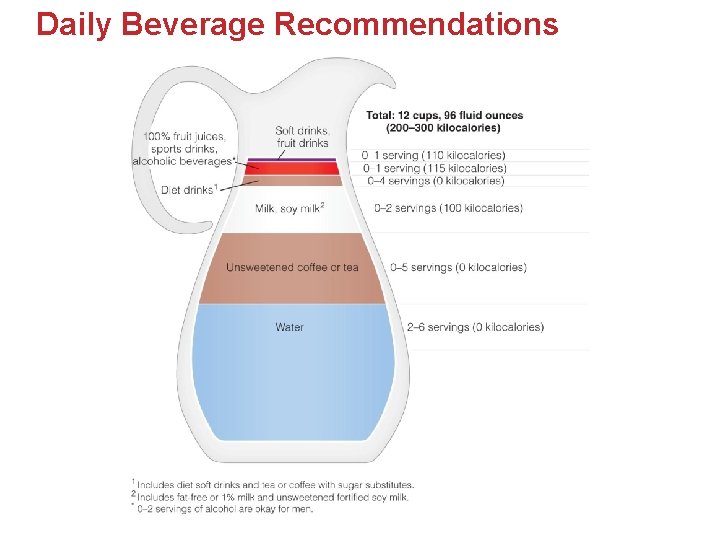
Daily Beverage Recommendations

Water Sources • Drink bottled or tap water, milk, and juices throughout the day. Let’s talks about this… • Most foods can also contribute to meeting daily water needs. • Fruits and vegetables can be 70% or more water by weight. • Dry grain products provide some water. • Cooking actually often adds water to food.

Drugs in Your Drinking Water Prescription Drugs (Prozac, Zoloft, Statins…). Antibiotics (for people and animals). Birth Control (Synthetic Female Sex Hormones). Rocket Fuel – Perchlorate Interrupt thyroid hormones, needed for pre- and postnatal development. Hypothyroidism. Heavy Metals - mercury, arsenic, chromium, cadmium, nickel and lead. These are all toxic to the human body. Chlorine – toxic cell killer! (trihalomethanes + Cl- = carcinogen). Fluoride - toxic to living organisms, leads to fluorosis.
Diuretics – increase urine output • Caffeine: • Mild diuretic that constricts blood vessels in the kidneys. • Some people are more sensitive to caffeine than others. • Tolerance to it’s diuretic effects.

Alcohol Will Dehydrate • Inhibits ADH (interfering with water balance) • Can induce urination as quickly as 20 minutes after consumption • Causes Dehydration • Affects electrolyte concentration, especially K+ Alcohol is also a CNS Depressant Cerebral Cortex: Uninhibited or Impaired judgment Hippocampus: Prevents short-term memories from becoming longterm; may experience exaggerated emotions and may black out Cerebellum: Individual loses hand-eye (leg) coordination Brain Stem: Impaired breathing and heart rate
Diuretic Medications Can Help Treat Hypertension • First line of treatment for hypertension • Often pharmaceutical diuretics • Promote diuresis by inhibiting the reabsorption of sodium – Increased sodium excretion increases fluid excretion. – Reduces blood volume and lowers blood pressure • Some diuretics increase potassium loss and the risk of hypokalemia.

Consuming Too Much Water Can Cause Hyponatremia Water Intoxication • Drinking fluid too fast without adequate Na+ replacement depletes Na+ and increases the rate of urine production. • Results in Hyponatremia = not enough Na+ • Can cause swelling in the brain and death! • Symptoms include fatigue, confusion, and disorientation, unconsciousness. . .

Consuming Too Little Water • Dehydration can be caused by: • Inadequate Water Intake • Strenuous Exercise in the Heat • Losing Excessive amounts of water from: diarrhea, vomiting, high fever, use of diuretics • As little as a 2% loss of body water can trigger: • Loss of short- and long-term memory • Lower attention span and cognition (lethargy) • Reduced ability to maintain core temperature • Increased risk of fatigue
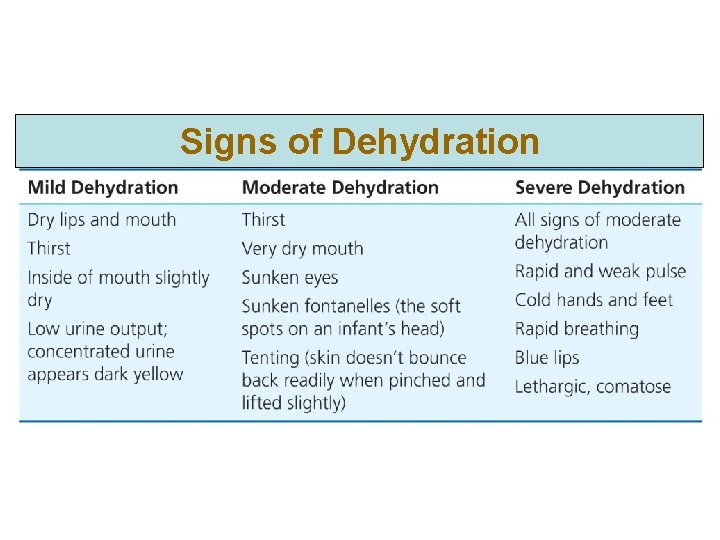
Signs of Dehydration
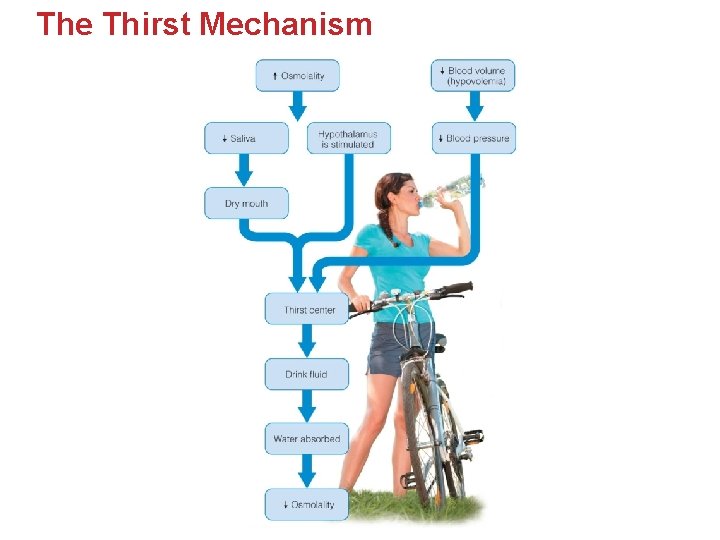
The Thirst Mechanism

• • Thirst often first sign of dehydration. Water lost from the body causes: • Reduced Blood Volume • Reduced Blood Pressure • • – – – • As a Consequence… if severe enough leads to Hypotension Reduced Cardiac Output Impaired Digestion Fainting or blackout Water depleted from ECF and ICF
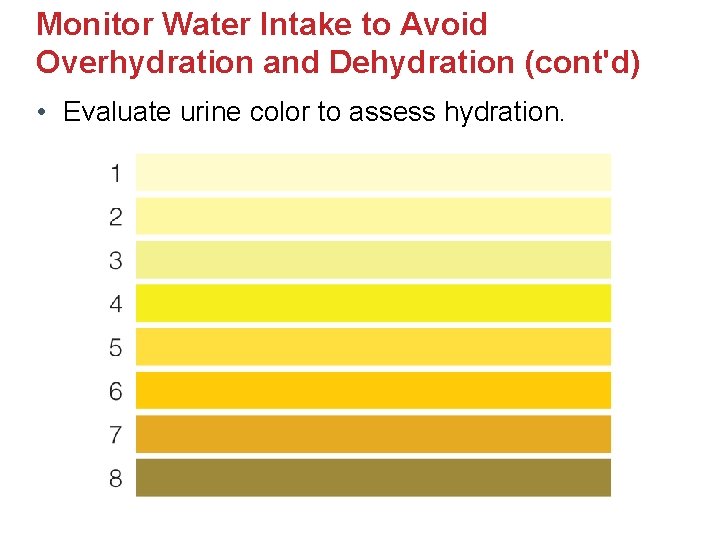
Monitor Water Intake to Avoid Overhydration and Dehydration (cont'd) • Evaluate urine color to assess hydration.
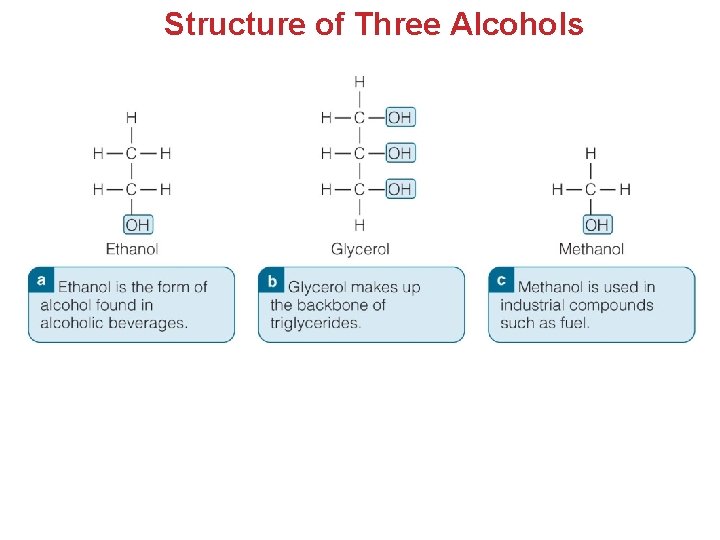
Structure of Three Alcohols
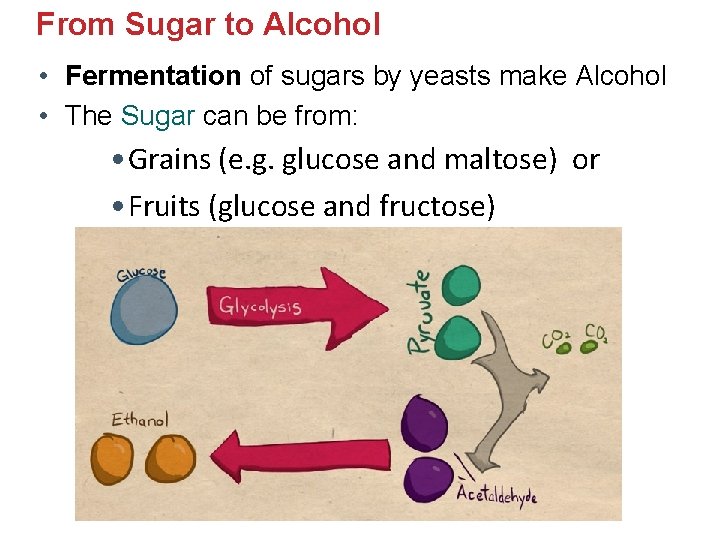
From Sugar to Alcohol • Fermentation of sugars by yeasts make Alcohol • The Sugar can be from: • Grains (e. g. glucose and maltose) or • Fruits (glucose and fructose)
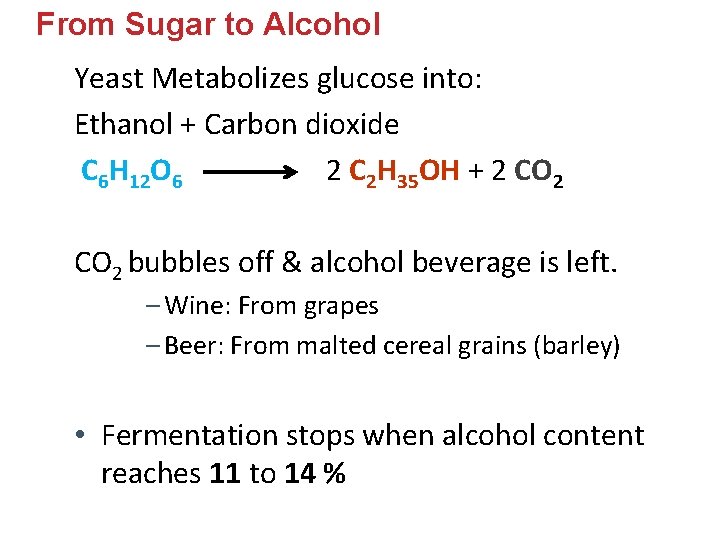
From Sugar to Alcohol Yeast Metabolizes glucose into: Ethanol + Carbon dioxide C 6 H 12 O 6 2 C 2 H 35 OH + 2 CO 2 bubbles off & alcohol beverage is left. – Wine: From grapes – Beer: From malted cereal grains (barley) • Fermentation stops when alcohol content reaches 11 to 14 %
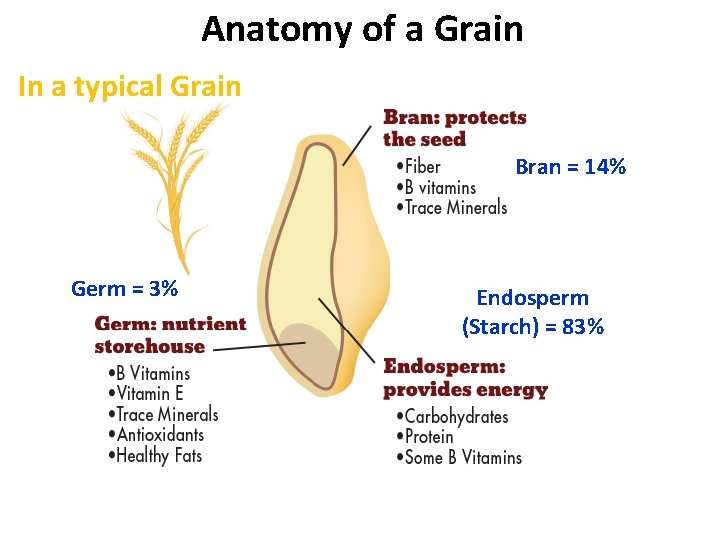
Anatomy of a Grain In a typical Grain Bran = 14% Germ = 3% Endosperm (Starch) = 83%
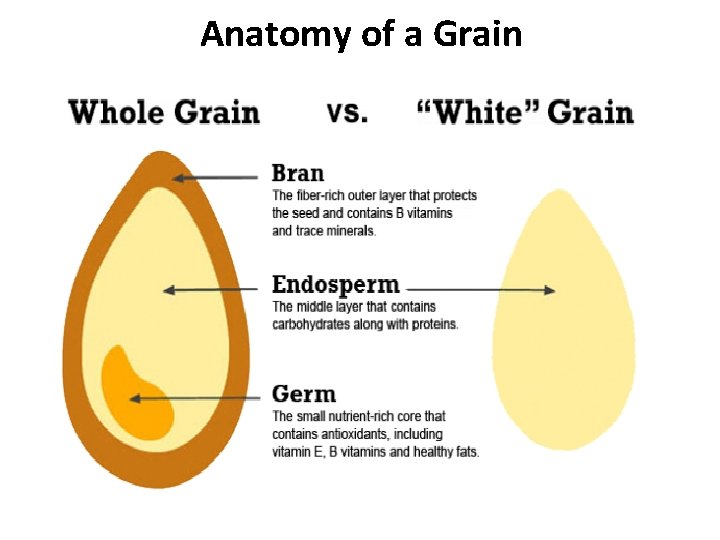
Anatomy of a Grain

Wine from fermentation of natural sugars in grapes. Cider is from apples; Perry from pears; Mead from honey. Brandy from distillation of fruit-fermented drinks (involves Evaporation and Concentration of alcohol content) Beer, Whiskey, and Vodka are produced by fermentation of Grain Starches converted to sugar by amylase – naturally present in the kernels of the grain that have malted (i. e. , germinated). Potatoes and un-malted grain are also starches amylase will ferment; this can be added to the mixture. Whiskey and Vodka are also distilled. Gin made by adding flavoring during distillation. Sake is rice wine involves fermentation mold Aspergillus oryzae. Rum by fermentation then distillation of sugarcane (molasses).
- Slides: 64