Water as a Solvent When something dissolves it








- Slides: 21

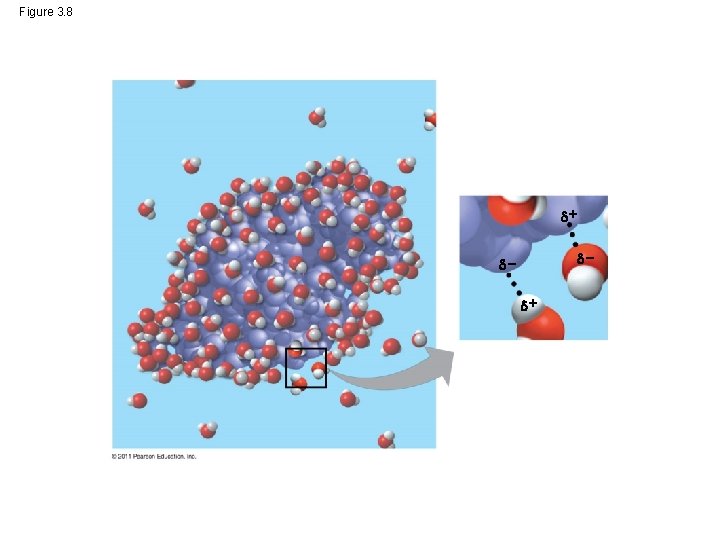
Water as a Solvent • When something dissolves it is called a solute • The solvent is the liquid (e. g. water) that it dissolves in. • Together, they are a solution. • Many things, even many proteins can dissolve in water. © 2011 Pearson Education, Inc.

Figure 3. 8 +

Dissociation of water • A hydrogen atom in a hydrogen bond between two water molecules can shift from one to the other – The molecule that lost the H+ is now a hydroxide ion (OH–) – The H+ is called a hydrogen ion © 2011 Pearson Education, Inc.

Individual Activity • H+ ion

Dissociation of water • A hydrogen atom in a hydrogen bond between two water molecules can shift from one to the other – The molecule that lost the H+ is now a hydroxide ion (OH–) – The H+ is called a hydrogen ion (or proton) • Water is in a state of dynamic equilibrium in which water molecules dissociate at the same rate at which they are being reformed © 2011 Pearson Education, Inc.

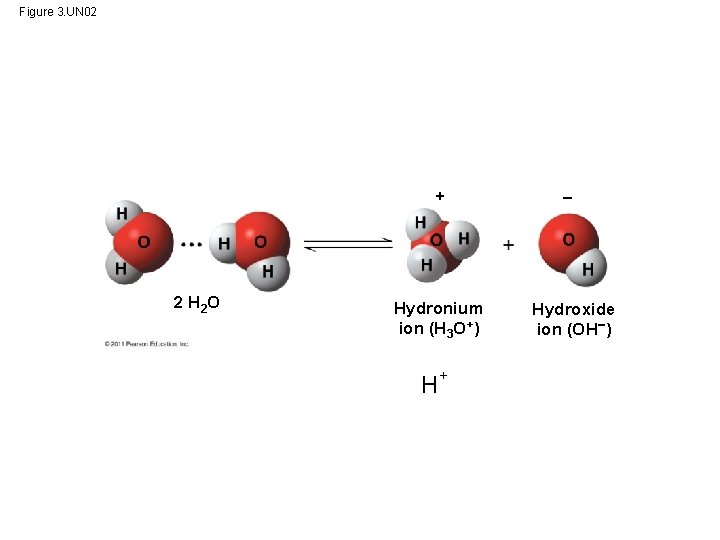
Figure 3. UN 02 + 2 H 2 O Hydronium ion (H 3 O+) H+ Hydroxide ion (OH )

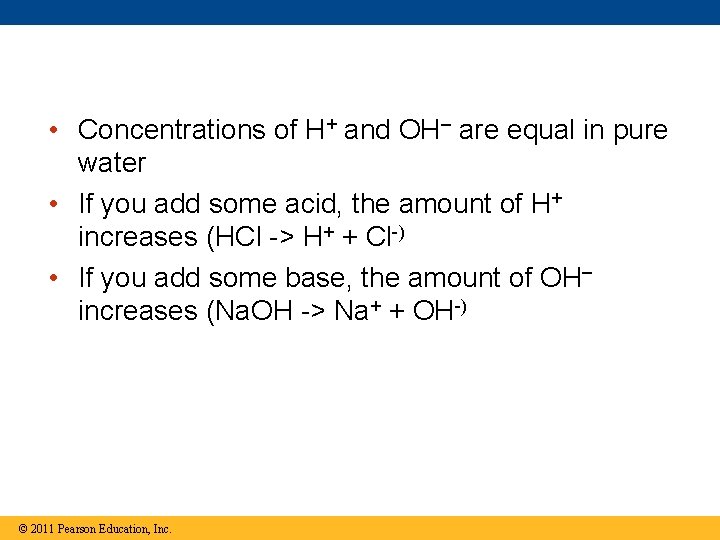
• Concentrations of H+ and OH– are equal in pure water • If you add some acid, the amount of H+ increases (HCl -> H+ + Cl-) • If you add some base, the amount of OH– increases (Na. OH -> Na+ + OH-) © 2011 Pearson Education, Inc.

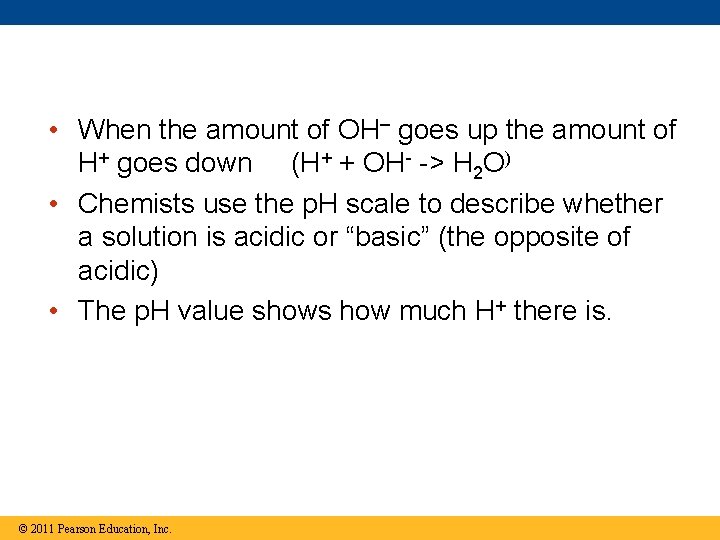
• When the amount of OH– goes up the amount of H+ goes down (H+ + OH- -> H 2 O) • Chemists use the p. H scale to describe whether a solution is acidic or “basic” (the opposite of acidic) • The p. H value shows how much H+ there is. © 2011 Pearson Education, Inc.

Acids and Bases • An acid is any substance that increases the H+ concentration of a solution • A base is any substance that reduces the H+ concentration of a solution © 2011 Pearson Education, Inc.

Objective 1: … about two models of acids and bases and the relationship of conjugate acid-base pairs

Arrhenius Theory • Acids ionize in water to H+ ions and anions • Bases ionize in water to OH- ions and cations • Neutralization reaction involves H+ combining with OH- to make water

Arrhenius Theory (cont. ) • Definition does not explain why ammonia solutions turn litmus blue – Basic without OH- ions

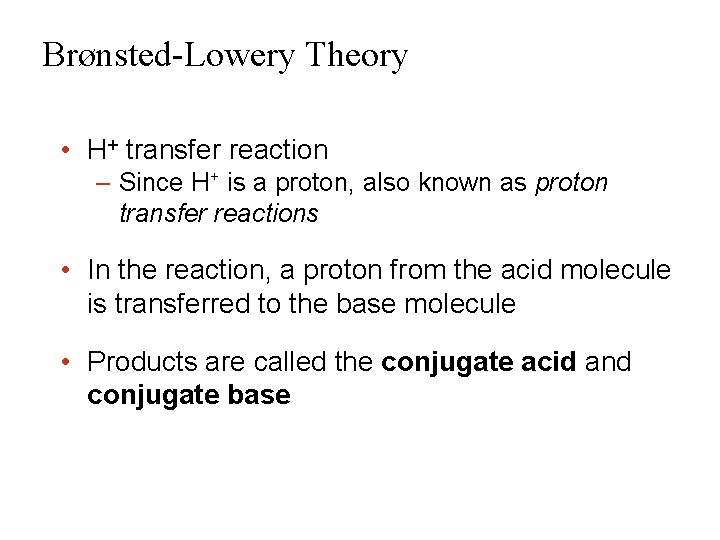
Brønsted-Lowery Theory • H+ transfer reaction – Since H+ is a proton, also known as proton transfer reactions • In the reaction, a proton from the acid molecule is transferred to the base molecule • Products are called the conjugate acid and conjugate base

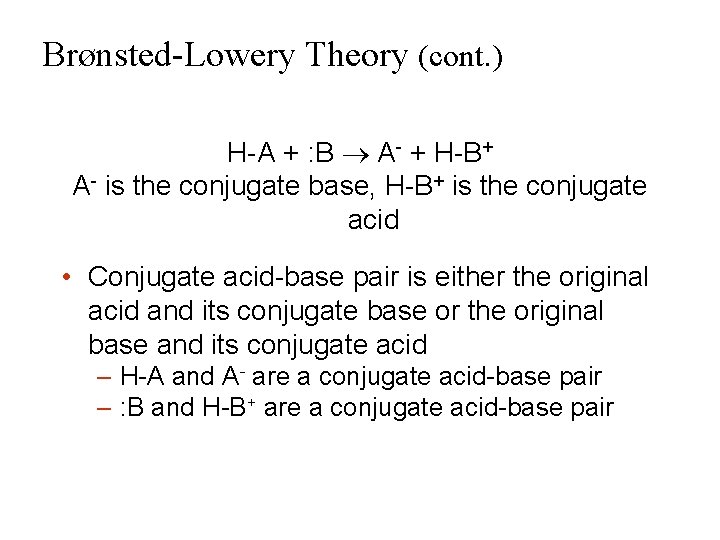
Brønsted-Lowery Theory (cont. ) H-A + : B A- + H-B+ A- is the conjugate base, H-B+ is the conjugate acid • Conjugate acid-base pair is either the original acid and its conjugate base or the original base and its conjugate acid – H-A and A- are a conjugate acid-base pair – : B and H-B+ are a conjugate acid-base pair

Example #1: Write the conjugate base for the acid H 3 PO 4 • Determine what species you will get if you remove 1 H+ from the acid. – Conjugate base will have one more negative charge than the original acid H 3 PO 4 H+ + H 2 PO 4 -

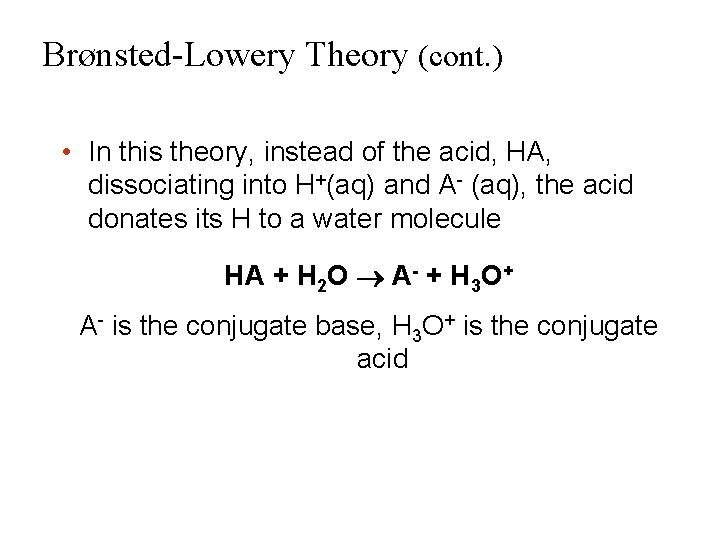
Brønsted-Lowery Theory (cont. ) • In this theory, instead of the acid, HA, dissociating into H+(aq) and A- (aq), the acid donates its H to a water molecule HA + H 2 O A- + H 3 O+ A- is the conjugate base, H 3 O+ is the conjugate acid

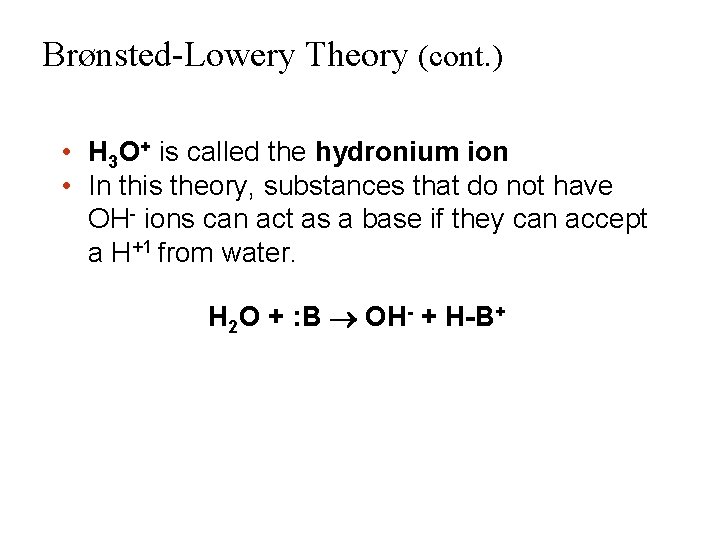
Brønsted-Lowery Theory (cont. ) • H 3 O+ is called the hydronium ion • In this theory, substances that do not have OH- ions can act as a base if they can accept a H+1 from water. H 2 O + : B OH- + H-B+

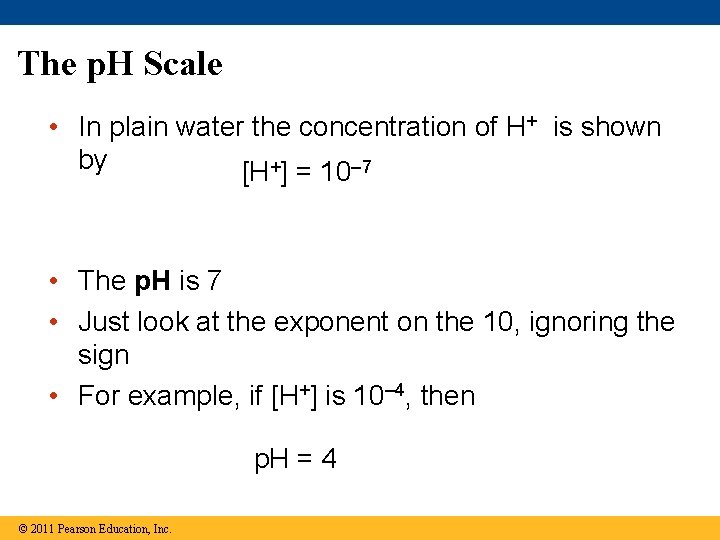
The p. H Scale • In plain water the concentration of H+ is shown by [H+] = 10– 7 • The p. H is 7 • Just look at the exponent on the 10, ignoring the sign • For example, if [H+] is 10– 4, then p. H = 4 © 2011 Pearson Education, Inc.

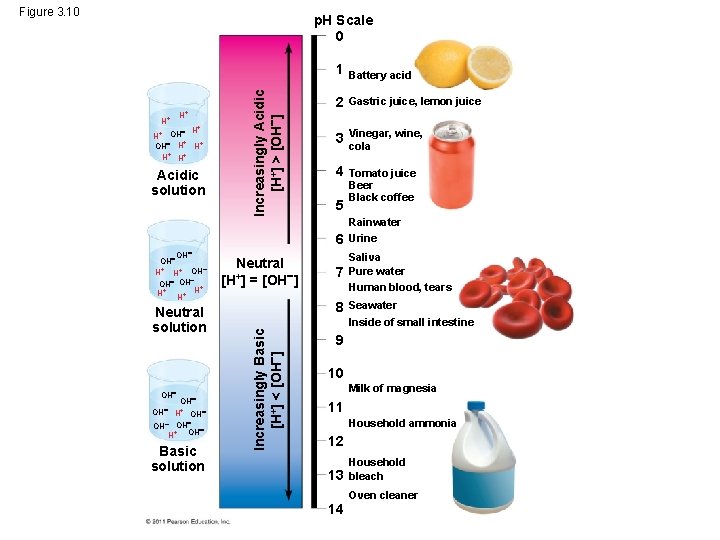
• Acidic solutions have p. H values less than 7 • Basic solutions have p. H values greater than 7 © 2011 Pearson Education, Inc.

Figure 3. 10 H+ H+ + OH H H+ H+ Acidic solution Increasingly Acidic [H+] > [OH ] p. H Scale 0 1 Battery acid 2 Gastric juice, lemon juice 3 Vinegar, wine, cola 4 Tomato juice Beer Black coffee 5 6 OH H+ H+ OH OH + H+ H+ H Neutral solution OH OH H+ OH OH H+ OH Basic solution Neutral [H+] = [OH ] 7 8 Increasingly Basic [H+] < [OH ] OH Rainwater Urine Saliva Pure water Human blood, tears Seawater Inside of small intestine 9 10 Milk of magnesia 11 Household ammonia 12 13 14 Household bleach Oven cleaner

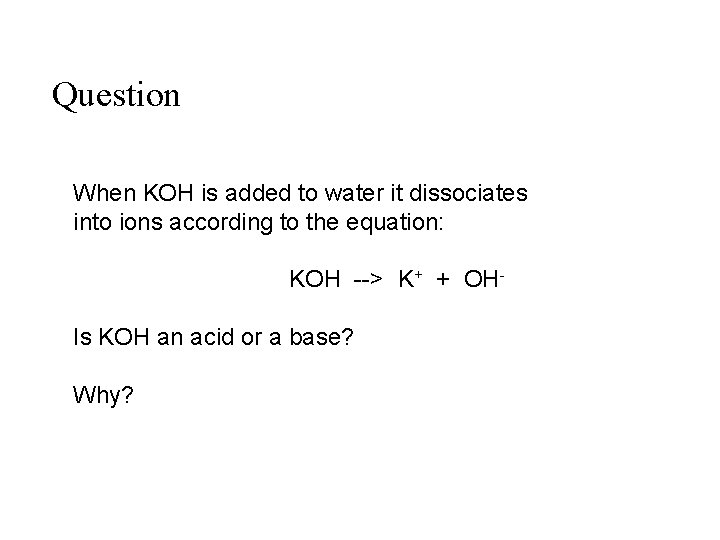
Question When KOH is added to water it dissociates into ions according to the equation: KOH --> K+ + OHIs KOH an acid or a base? Why?