WATER AND THE SOLUTION PROCESS A A water


















- Slides: 19

WATER AND THE SOLUTION PROCESS A. A water molecule consists of two atoms of _____ hydrogen and one atom of _______ oxygen united by _______ polar covalent bonds.

WATER AND THE SOLUTION PROCESS 1. The _____ polar nature of the water molecule allows for hydrogen bonding between ____ water molecules which contributes to some of water’s unique properties.

WATER AND THE SOLUTION PROCESS a. _______ Surface ______ tensionis the inward force or pull that tends surface ____ area to minimize the _______ of a liquid. Water has a relatively ____ high surface tension.

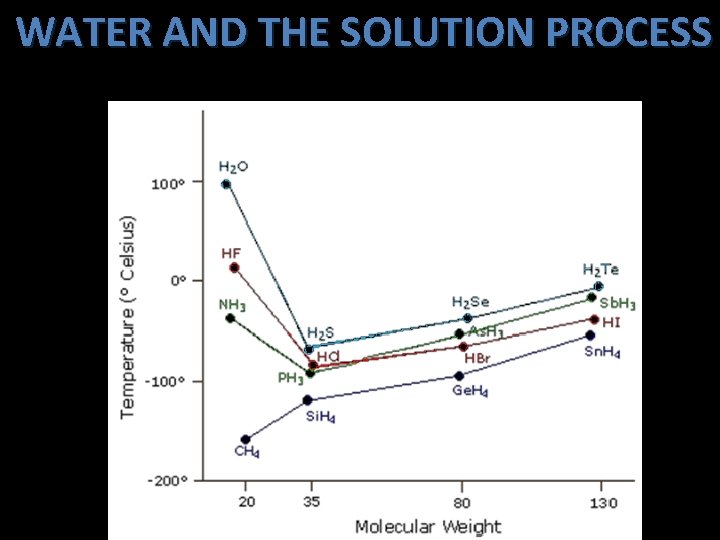
WATER AND THE SOLUTION PROCESS b. Water has a relatively _____ high boiling point due to the hydrogen bonding extensive ____ that occurs between water molecules.

WATER AND THE SOLUTION PROCESS

WATER AND THE SOLUTION PROCESS c. Hydrogen bonding holds water molecules in a hexagonal ____ arrangement in the solid form. This allows the solid form of water to occupy a greater volume and therefore smaller ______. density

WATER AND THE SOLUTION PROCESS aqueous solution is A. An ____ water that contains ____ dissolved substances.

WATER AND THE SOLUTION PROCESS B. The solution process depends on the _____ force of attraction between the solute _____, the thing getting dissolved, and the _______, solvent the thing doing the dissolving.

H H +Dissoc iationionic compound made A. When an _____ of ____ ions dissolves in water, the ions separate ____ from one another. This is called ______ dissociation and is the δ- δ+ O O δ+ δ- H H result of the strong attraction between the ions and the polar water molecules

δ+ δ- H H +Dissoc iation- O O δ- H H δ+ 1. The result is a solution with free moving charged particles able to electricity conduct _____.

H H +Dissoc iationions produced in 2. The number of ____ solution depends upon the _____ ratio in formula the original ____. δ- δ+ O O δ+ δ- H H

H H +Dissoc iationcovalent compounds B. Most _____ δ- δ+ O O δ+ δ- H H (except acids), dissolve in water as intact molecules. _______

δ+ δ- H H +Dissoc iation- O O δ- H H δ+ A. A solution that dissolves in ions water to give a solution of ____ does conduct electric current and is electrolyte called an _____

H H +Dissoc iation 1. Typically, ionic _____ compounds will δ- δ+ O O δ+ δ- H H conduct electrical current when dissolved in water.

H H +Dissoc iation 2. Strong _____ acids also are good δ- H H electrolytes δ+ O O δ+ δ-

δ+ δ- H H +Dissoc iation- O O δ- H H δ+ B. A solute that dissolves in water molecules to give a solution of _____ does not conduct electric current nonelectrolyte and is called a _______.

H H +Dissoc iation 1. Typically, molecular _____ compounds δ- δ+ O O δ+ δ- H H will NOT conduct electrical current when dissolved in water.

δ+ δ- H H +Dissoc iation- O O δ- H H δ+ C. A solute that dissolves mostly as _____ but partially as ions ____ molecules does (although not as well) conduct electric current is called a _____ weak electrolyte

H H +Dissoc iation 1. Typically , weak _____ acids are δ- δ+ O O δ+ H H generally weak electrolytes δ-