WATER AND SOLUTIONS A solution is a mixture






- Slides: 16

WATER AND SOLUTIONS

A solution is a mixture of a solute and a solvent. • A solvent is a substance that dissolves other materials to form a solution e. g. Water, White Spirit, nail Varnish remover. A substance that dissolves in the solvent is called the solute e. g. coffee, salt, sugar.

SOLUTION solute + solvent >>>> solution Examples of Solutions • Sugar (solute) in water (solvent) • Copper sulfate (solute) in water (solvent) • Sea water


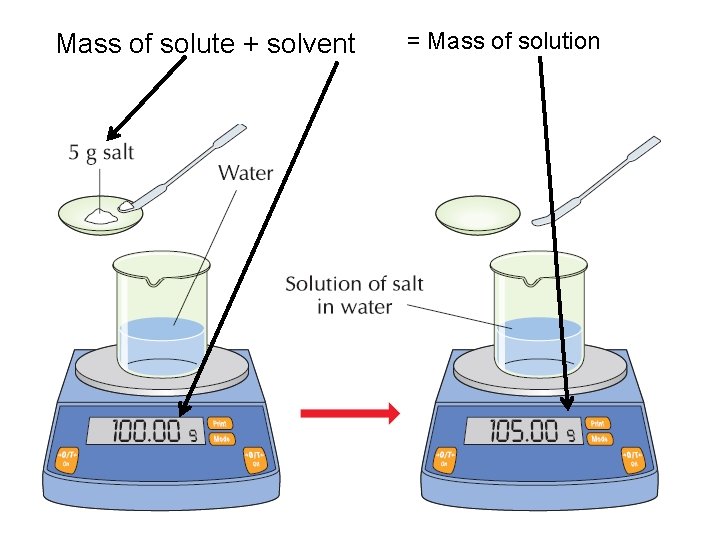
Mass of solute + solvent = Mass of solution

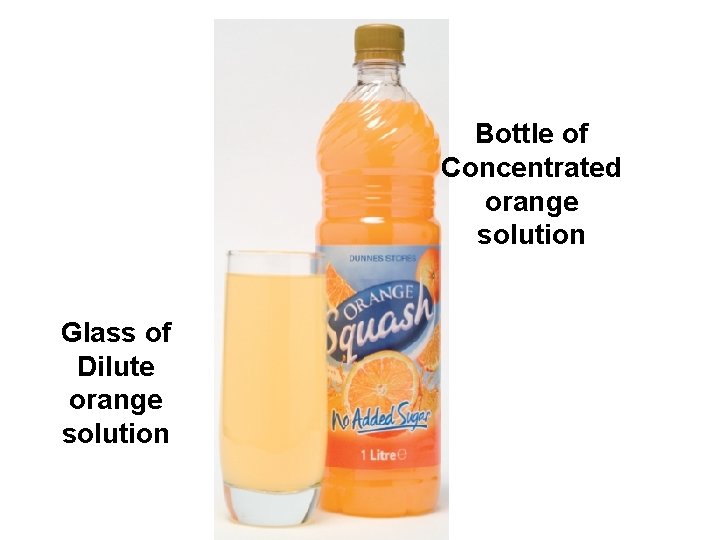
Bottle of Concentrated orange solution Glass of Dilute orange solution

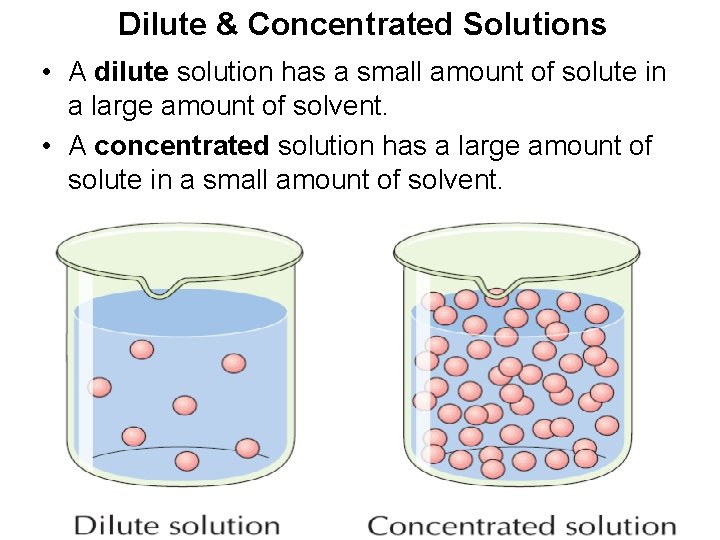
SOLUTIONS • A dilute solution has a small amount of solute in a large amount of solvent. • A concentrated solution has a large amount of solute in a small amount of solvent. Dilute solution of copper sulfate Concentrated solution of copper sulfate

Dilute & Concentrated Solutions • A dilute solution has a small amount of solute in a large amount of solvent. • A concentrated solution has a large amount of solute in a small amount of solvent.

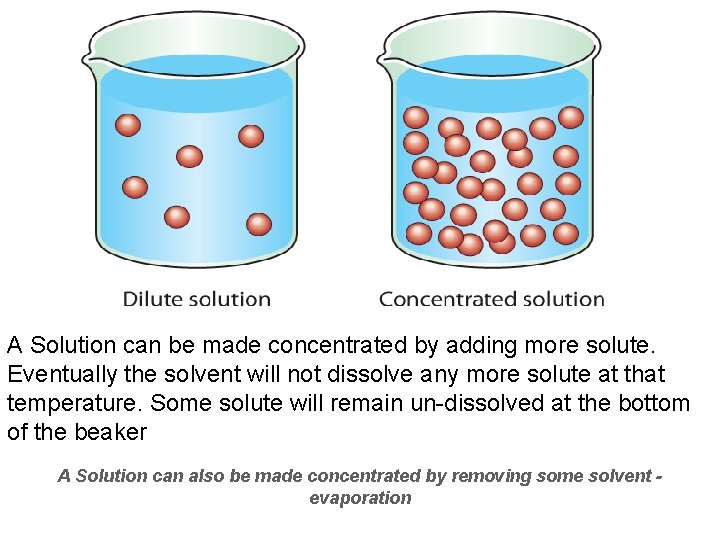
A Solution can be made concentrated by adding more solute. Eventually the solvent will not dissolve any more solute at that temperature. Some solute will remain un-dissolved at the bottom of the beaker A Solution can also be made concentrated by removing some solvent evaporation

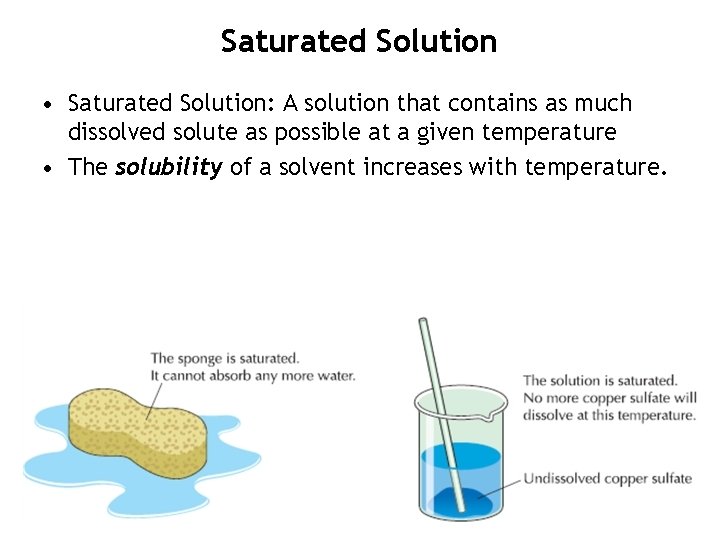
Saturated Solution • Saturated Solution: A solution that contains as much dissolved solute as possible at a given temperature • The solubility of a solvent increases with temperature.

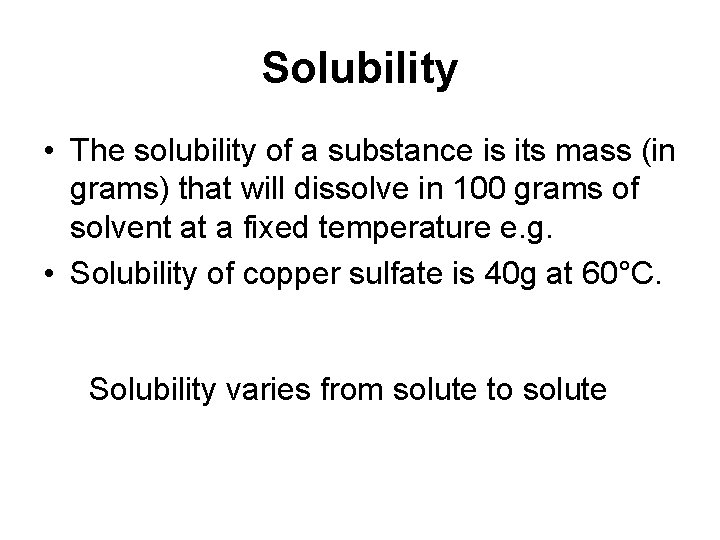
Solubility • The solubility of a substance is its mass (in grams) that will dissolve in 100 grams of solvent at a fixed temperature e. g. • Solubility of copper sulfate is 40 g at 60°C. Solubility varies from solute to solute

Solubility and Temperature In a concentrated solution some solute will remain undissolved at the bottom of the beaker. This solute can be made to dissolve by increasing the temperature of the solvent • The amount of solute that will dissolve in a solvent depends on the temperature e. g. • Coffee grains dissolve more easily in hot water than in cold water.

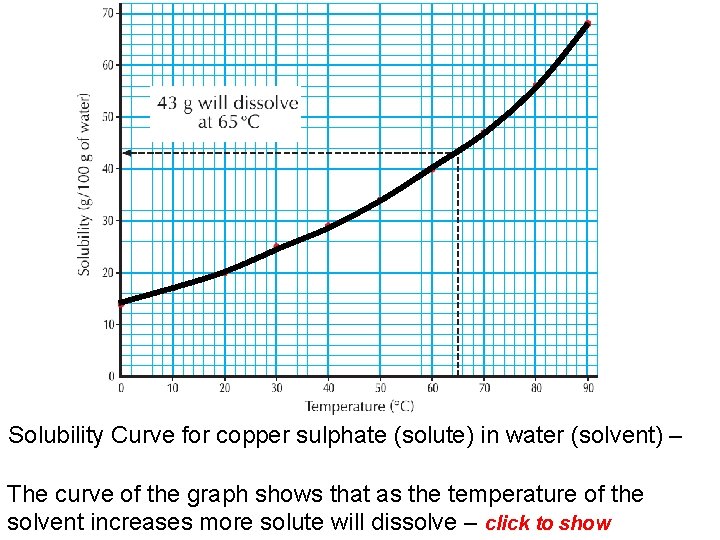
Solubility Curve for copper sulphate (solute) in water (solvent) – The curve of the graph shows that as the temperature of the solvent increases more solute will dissolve – click to show

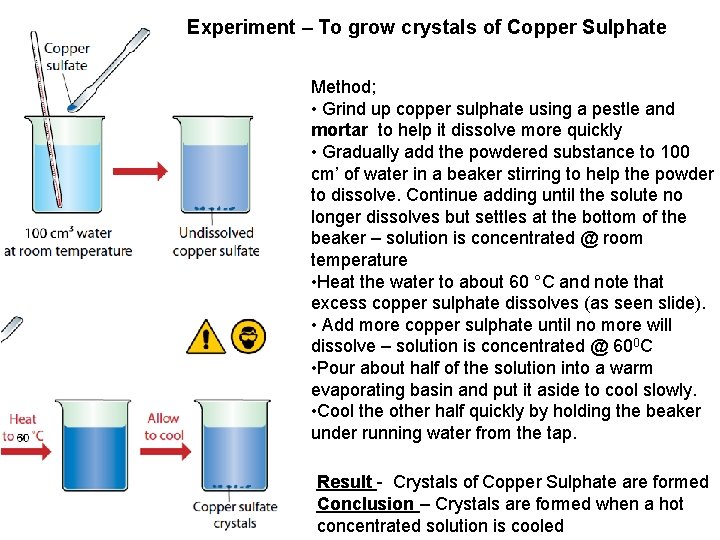
Experiment – To grow crystals of Copper Sulphate Method; • Grind up copper sulphate using a pestle and mortar to help it dissolve more quickly • Gradually add the powdered substance to 100 cm’ of water in a beaker stirring to help the powder to dissolve. Continue adding until the solute no longer dissolves but settles at the bottom of the beaker – solution is concentrated @ room temperature • Heat the water to about 60 °C and note that excess copper sulphate dissolves (as seen slide). • Add more copper sulphate until no more will dissolve – solution is concentrated @ 600 C • Pour about half of the solution into a warm evaporating basin and put it aside to cool slowly. • Cool the other half quickly by holding the beaker under running water from the tap. Result - Crystals of Copper Sulphate are formed Conclusion – Crystals are formed when a hot concentrated solution is cooled

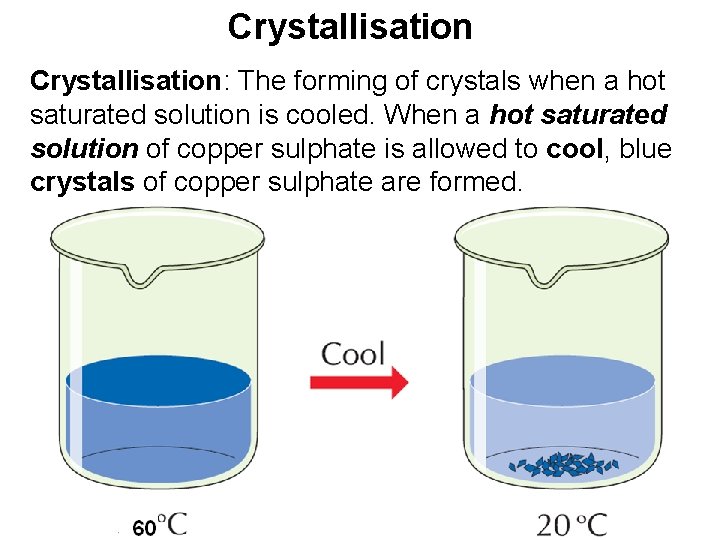
Crystallisation: The forming of crystals when a hot saturated solution is cooled. When a hot saturated solution of copper sulphate is allowed to cool, blue crystals of copper sulphate are formed.

END