Water and Ocean Structure WORLDS WATER SOURCES Learning







- Slides: 44

Water and Ocean Structure

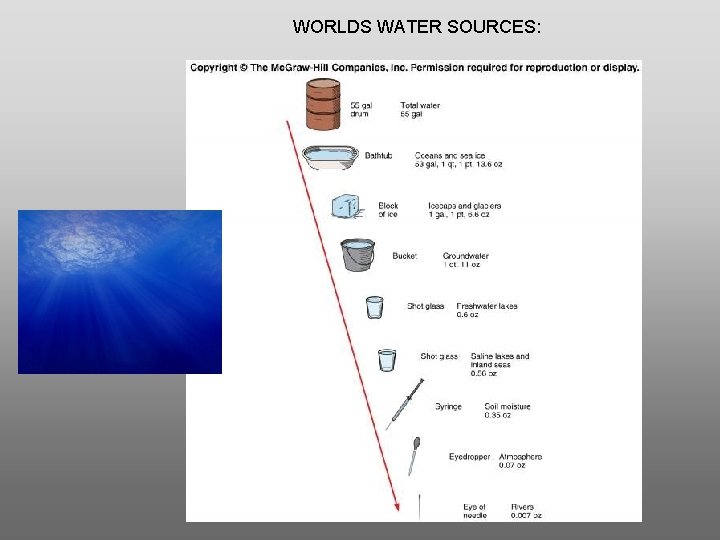
WORLDS WATER SOURCES:

Learning Objectives 1. Understand the nature of the water molecule and its unique properties (polarity, density and thermal properties) and how these are altered by the presence of salt in solution. 2. Know the types of materials that are dissolved in sea water, their importance and how they vary with time. 3. Explain variations in salinity, temperature, and pressure within the sea and how they alter the chemical and physical properties of the ocean.


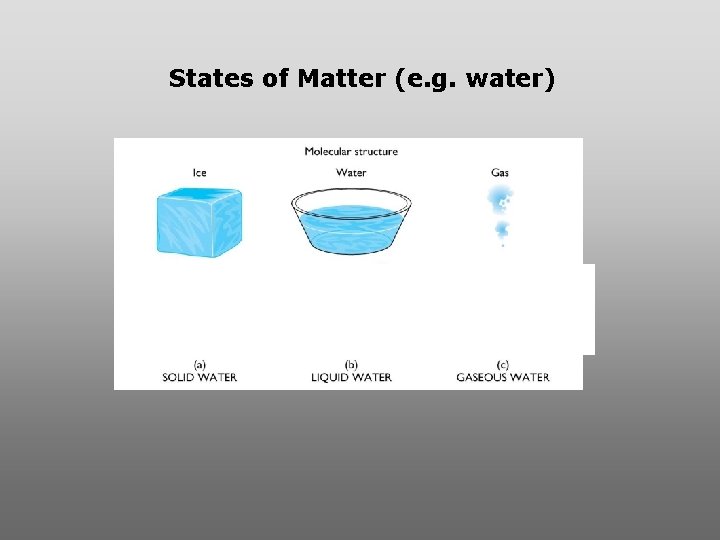
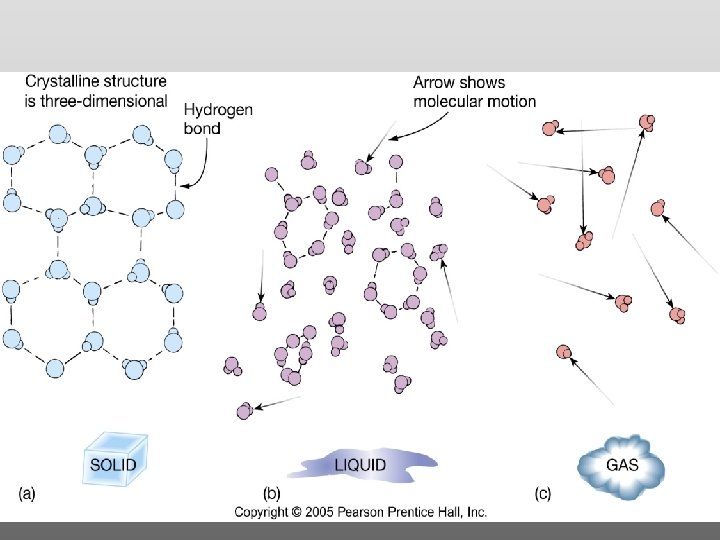
States of Matter (e. g. water)

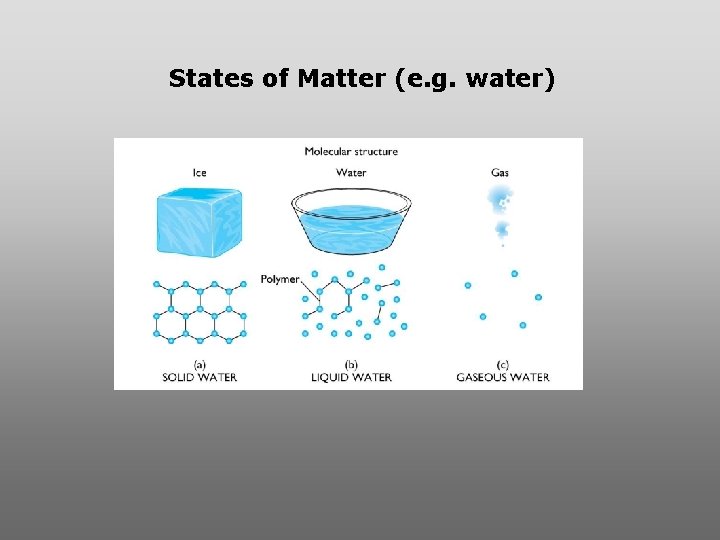
States of Matter (e. g. water)

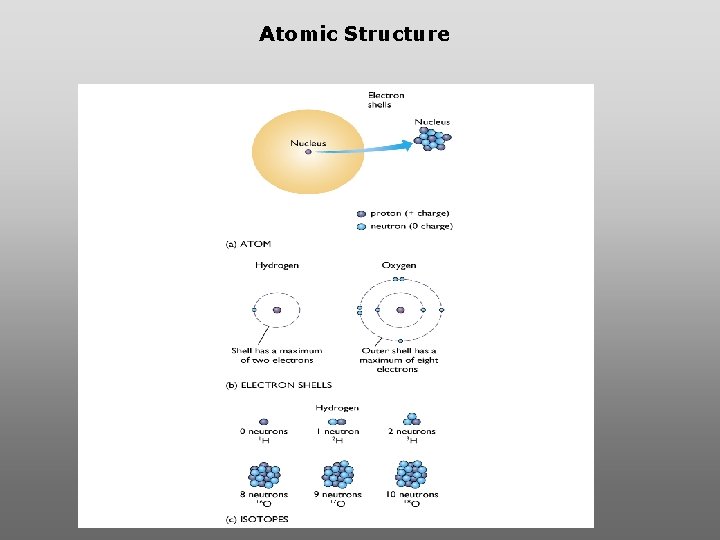
Atomic Structure

http: //www. dayah. com/periodic/Other/Periodic%20 Table. pdf

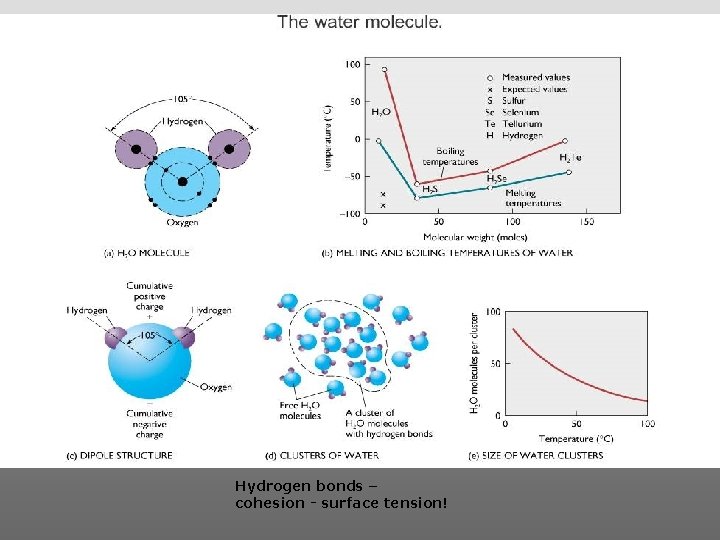
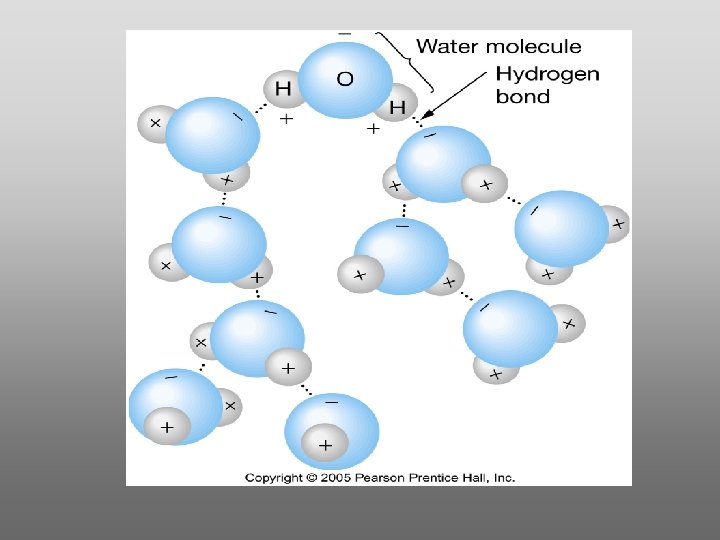
Hydrogen bonds – cohesion - surface tension!

http: //www-math. mit. edu/~dhu/Climberweb/climberweb. html


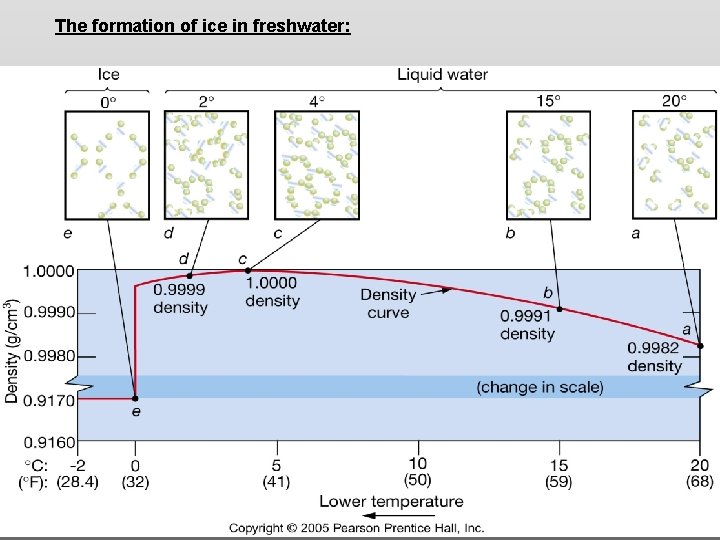
The formation of ice in freshwater:

Density of freshwater: Seawater density depends on temperature, salinity and pressure! Therefore, it increases with > salt content at const. temp; high density in cold, salty waters –why is this important?

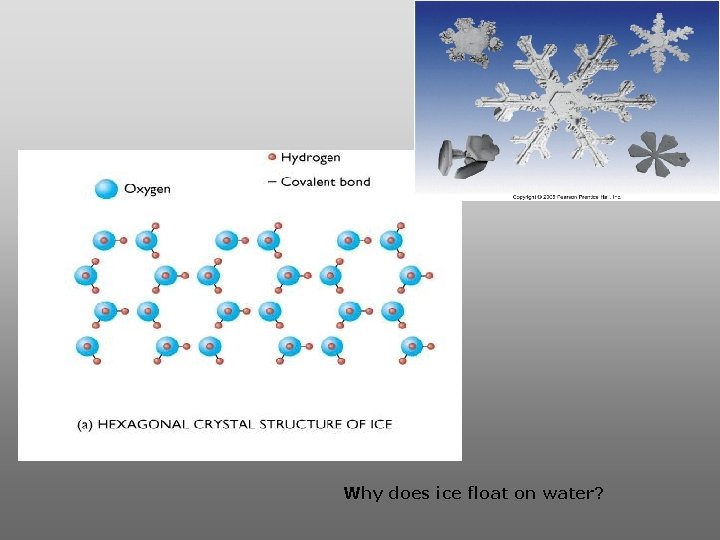
Why does ice float on water?


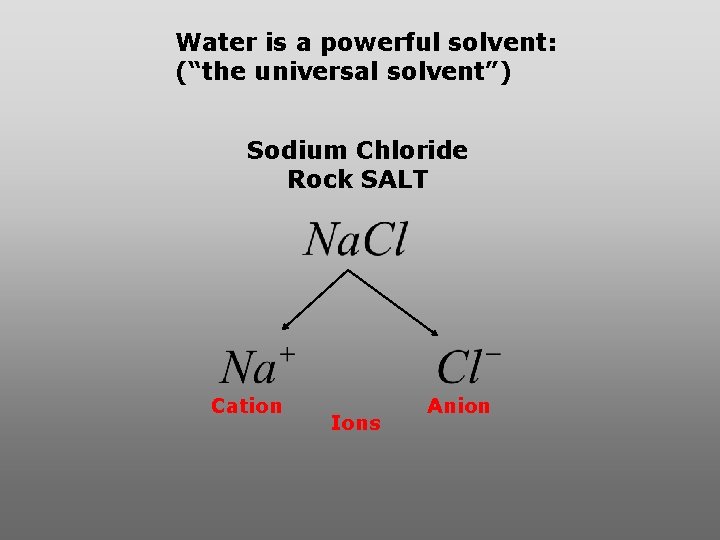
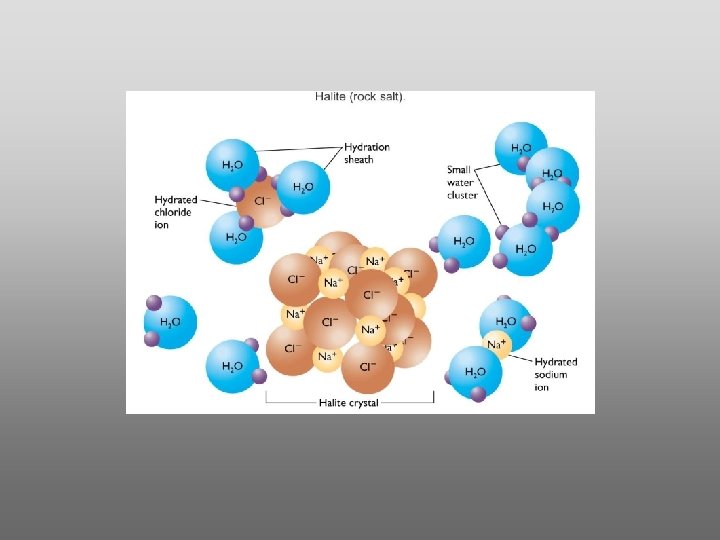
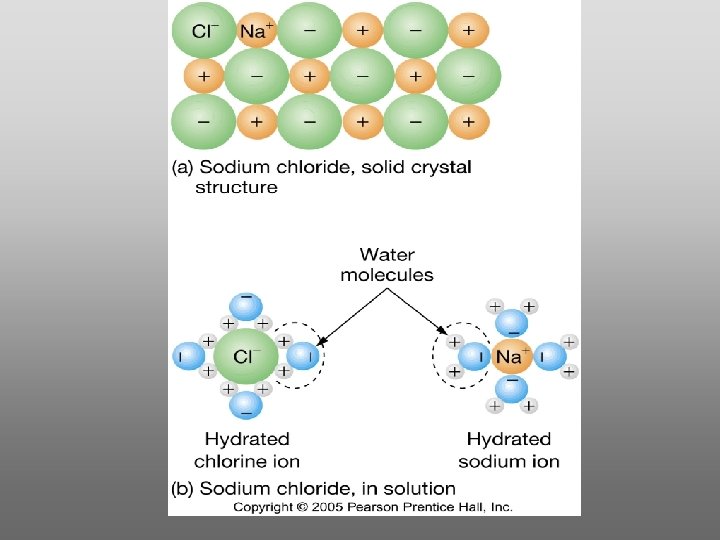
Water is a powerful solvent: (“the universal solvent”) Sodium Chloride Rock SALT Cation Ions Anion



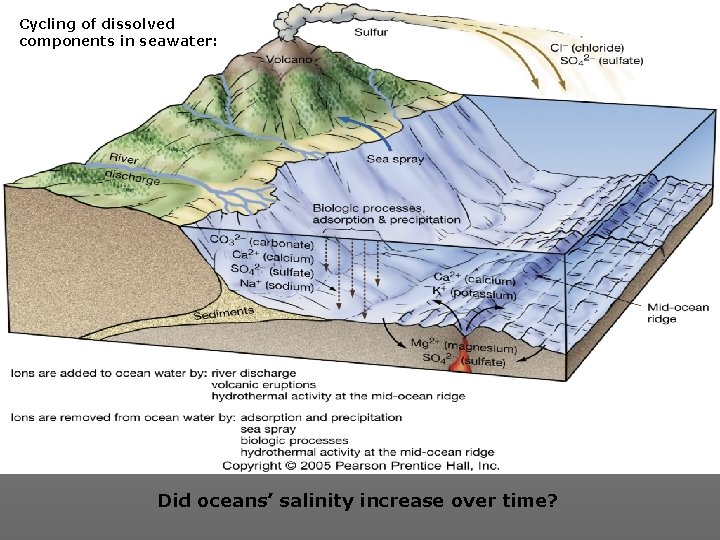
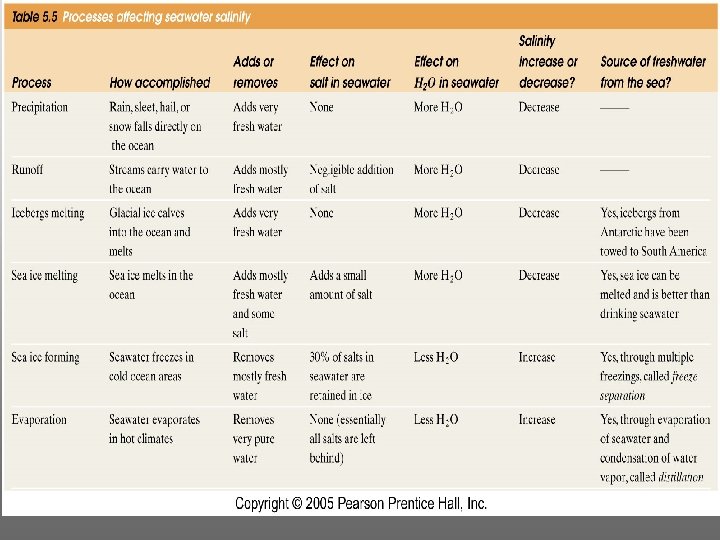
Cycling of dissolved components in seawater: Did oceans’ salinity increase over time?

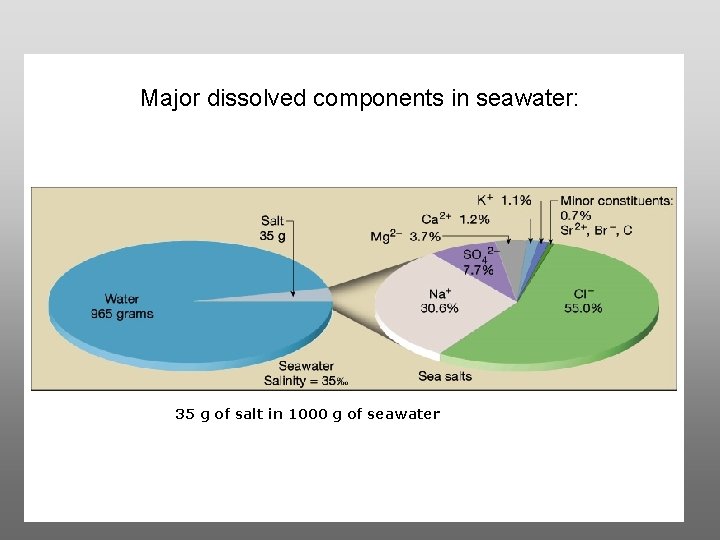
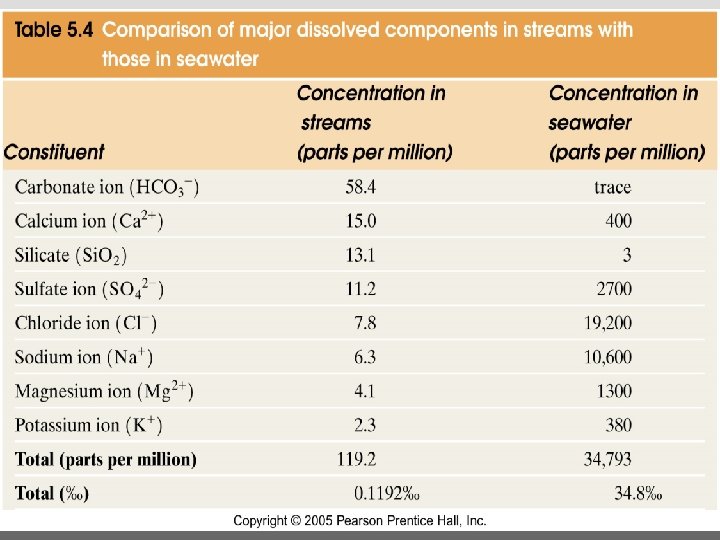
Major dissolved components in seawater: 35 g of salt in 1000 g of seawater

Residence Time FHow long do the various dissolved ions stay in the ocean? Depends on how “reactive” they are. FResidence Time: The average time spent by a substance in the Ocean = Amount in Sea Rate entering or exiting

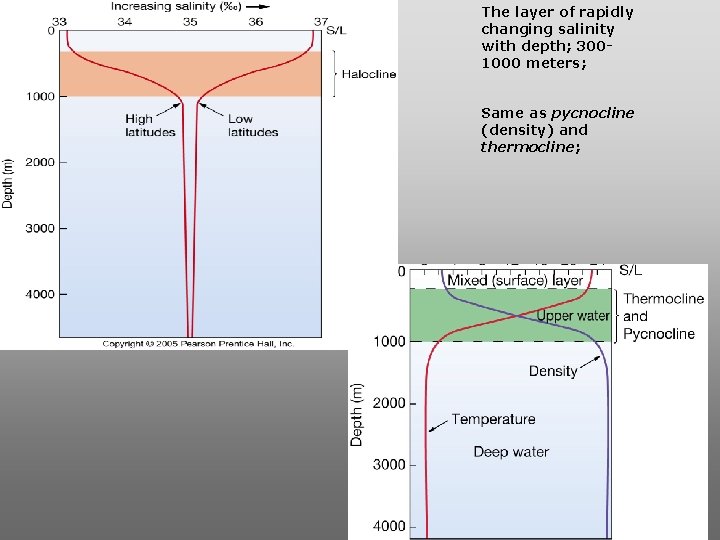
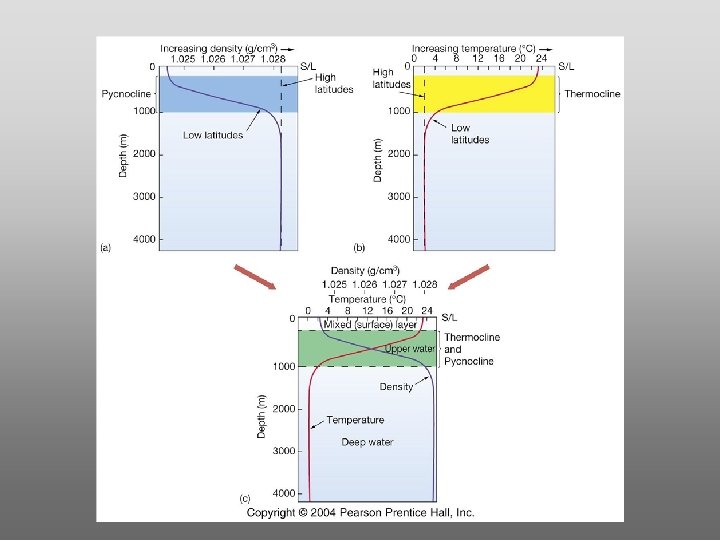
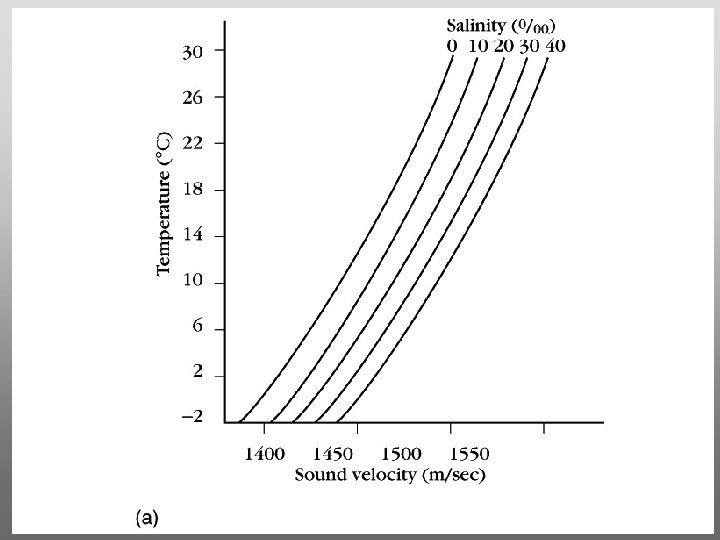
The layer of rapidly changing salinity with depth; 3001000 meters; Same as pycnocline (density) and thermocline;


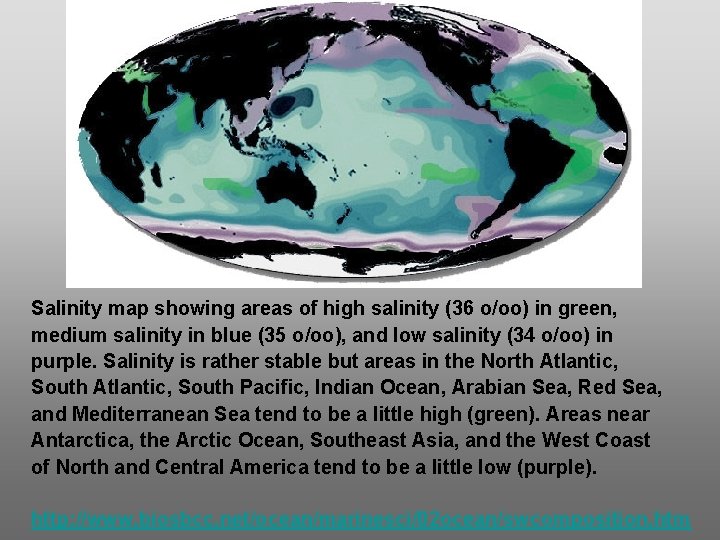
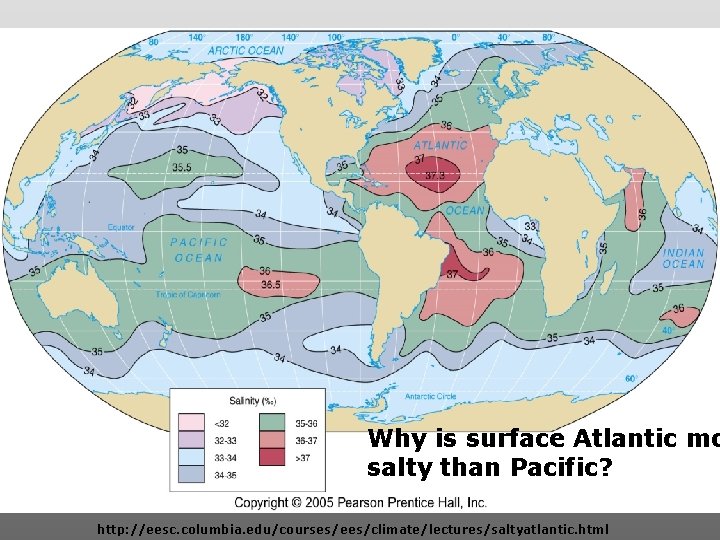
Salinity map showing areas of high salinity (36 o/oo) in green, medium salinity in blue (35 o/oo), and low salinity (34 o/oo) in purple. Salinity is rather stable but areas in the North Atlantic, South Pacific, Indian Ocean, Arabian Sea, Red Sea, and Mediterranean Sea tend to be a little high (green). Areas near Antarctica, the Arctic Ocean, Southeast Asia, and the West Coast of North and Central America tend to be a little low (purple). http: //www. biosbcc. net/ocean/marinesci/02 ocean/swcomposition. htm



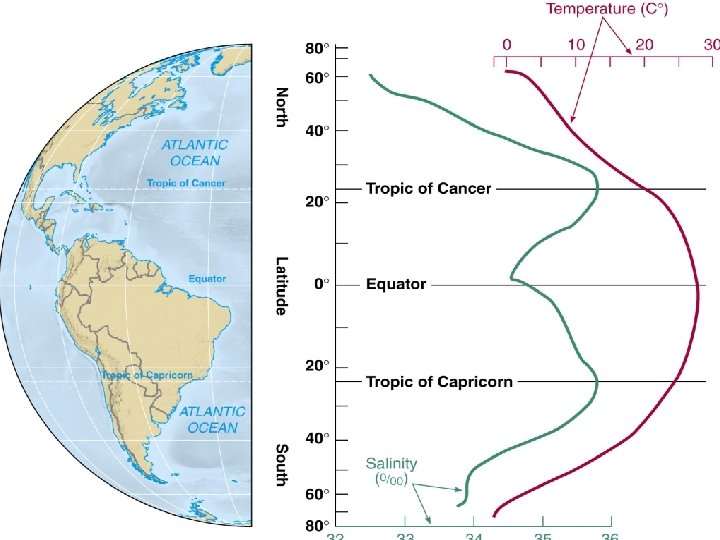
Why is surface Atlantic mo salty than Pacific? http: //eesc. columbia. edu/courses/ees/climate/lectures/saltyatlantic. html

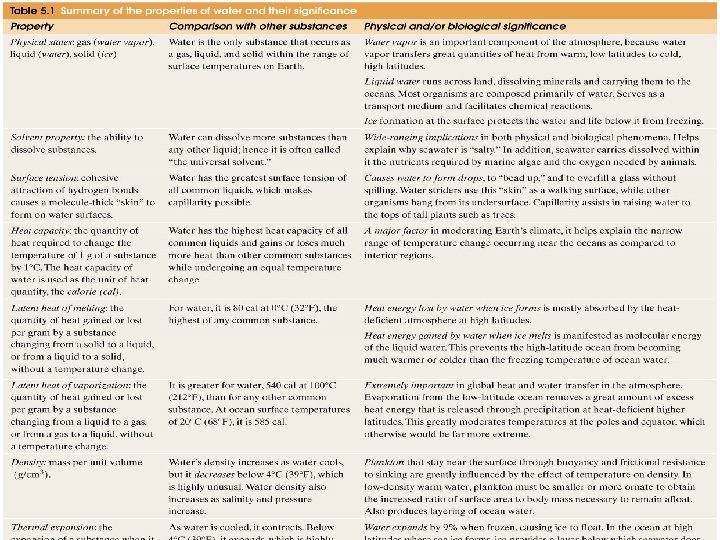
Summary: èWater is a polar molecule -- unique properties (melting pt, heat capacity, dissolving power, water denser than ice) èSalinity is the total dissolved solids èSalinity in the surface ocean varies by Evaporation - Precipitation èPrinciple of Constant Proportions èResidence Time in the Oceans


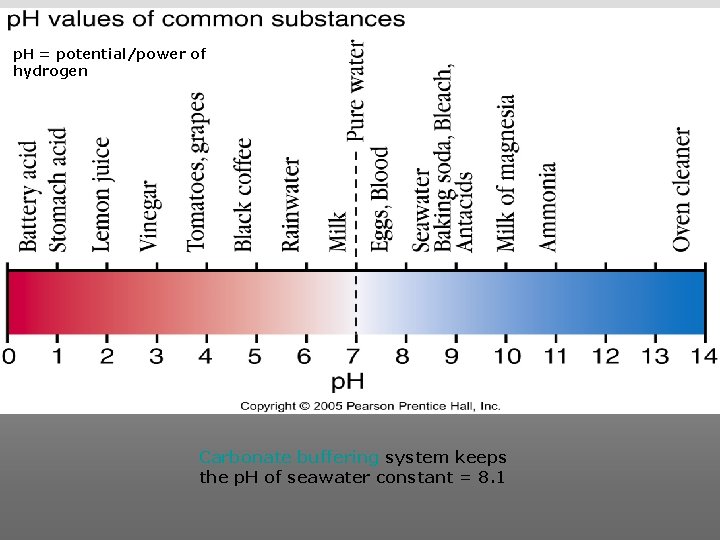
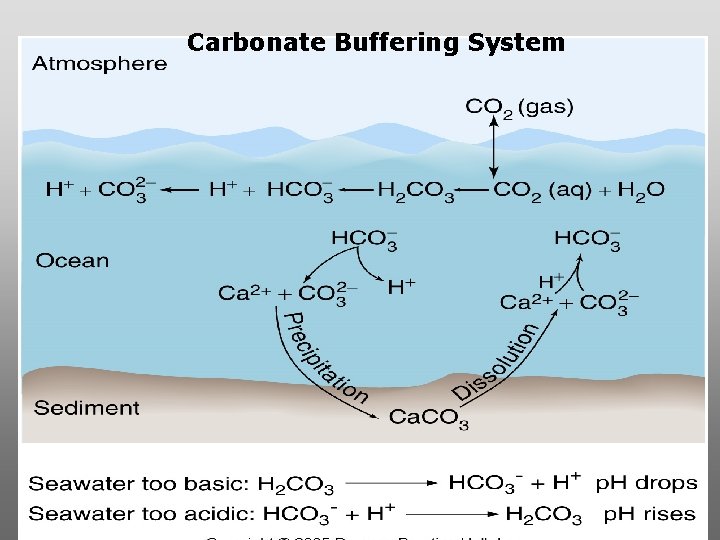
p. H = potential/power of hydrogen Carbonate buffering system keeps the p. H of seawater constant = 8. 1

Carbonate Buffering System

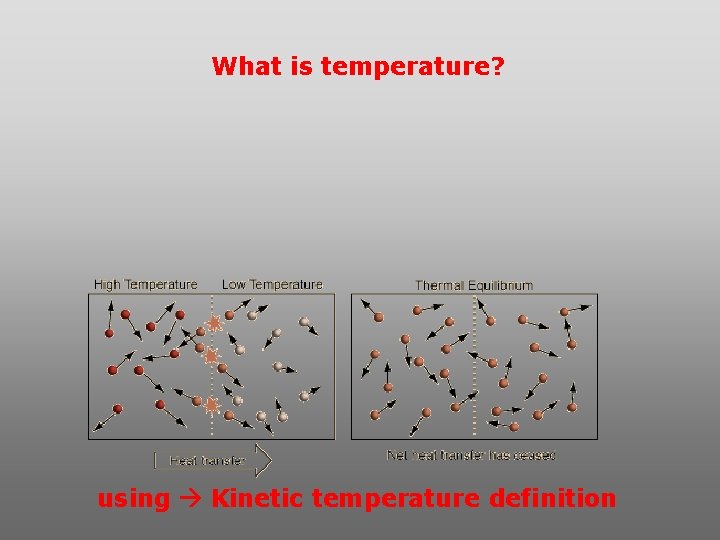
What is temperature? using Kinetic temperature definition

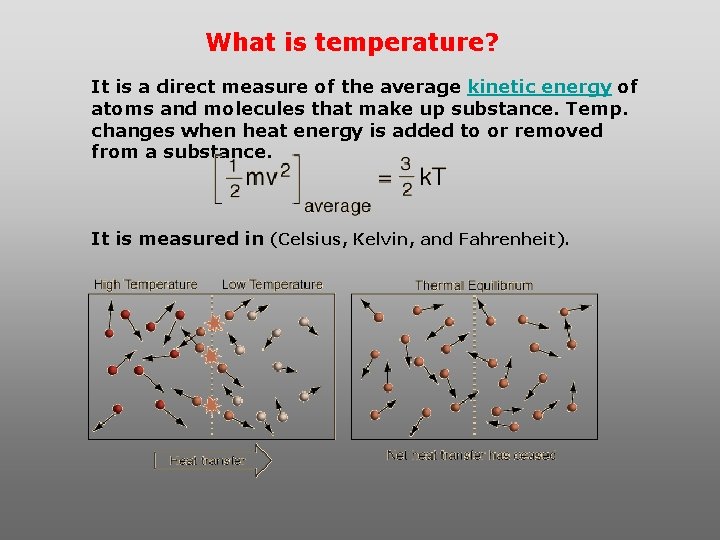
What is temperature? It is a direct measure of the average kinetic energy of atoms and molecules that make up substance. Temp. changes when heat energy is added to or removed from a substance. It is measured in (Celsius, Kelvin, and Fahrenheit).

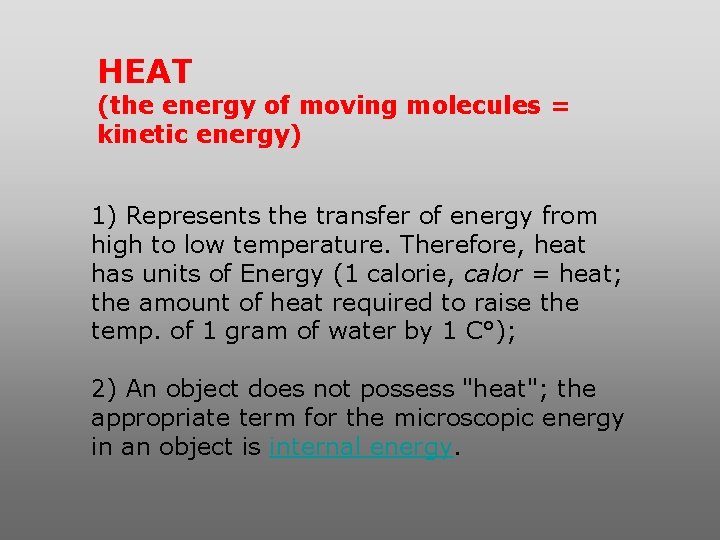
HEAT (the energy of moving molecules = kinetic energy) 1) Represents the transfer of energy from high to low temperature. Therefore, heat has units of Energy (1 calorie, calor = heat; the amount of heat required to raise the temp. of 1 gram of water by 1 C°); 2) An object does not possess "heat"; the appropriate term for the microscopic energy in an object is internal energy.

Temperature vs Heat FTemperature is a measure of how fast the molecules in a substance are moving FHeat is a measure of how much energy has to be put into (or gotten out of) a substance to change its temperature, or “state” (solid, liquid, gas)

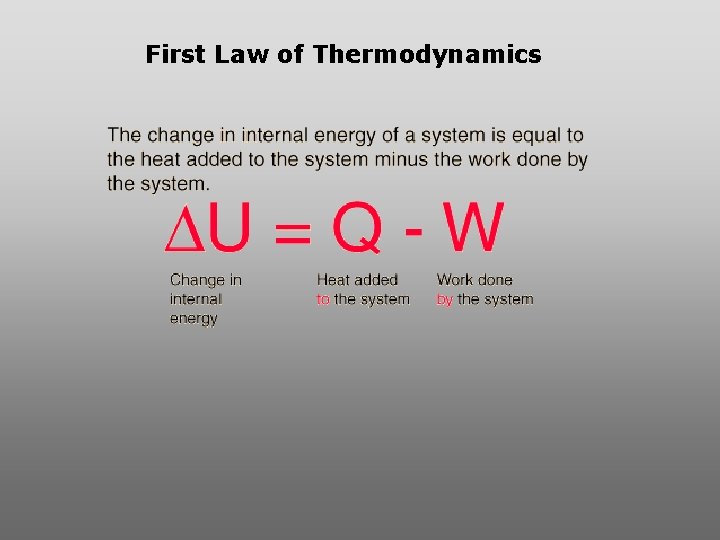
First Law of Thermodynamics

Heat Capacity – the amount of heat required to raise the temp. of 1 g of any substance by 1 °C; – Water has one of the highest heat capacities known, which makes water excellent heat transfer material; and – therefore, allows ocean currents to moderate global climate!


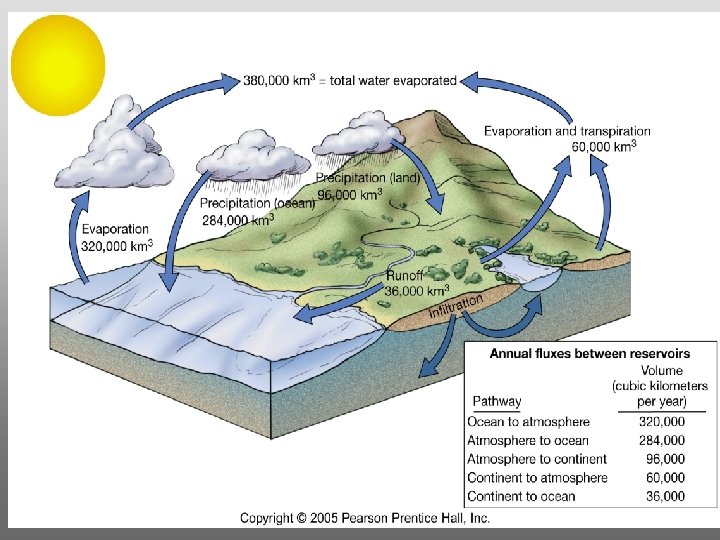
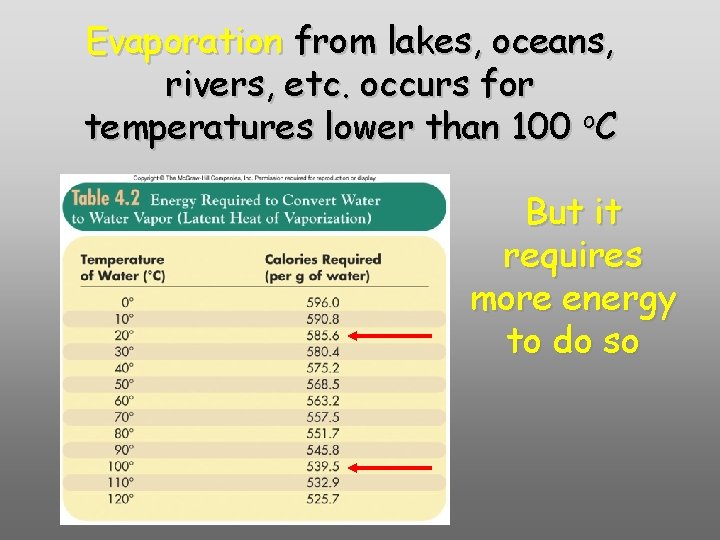
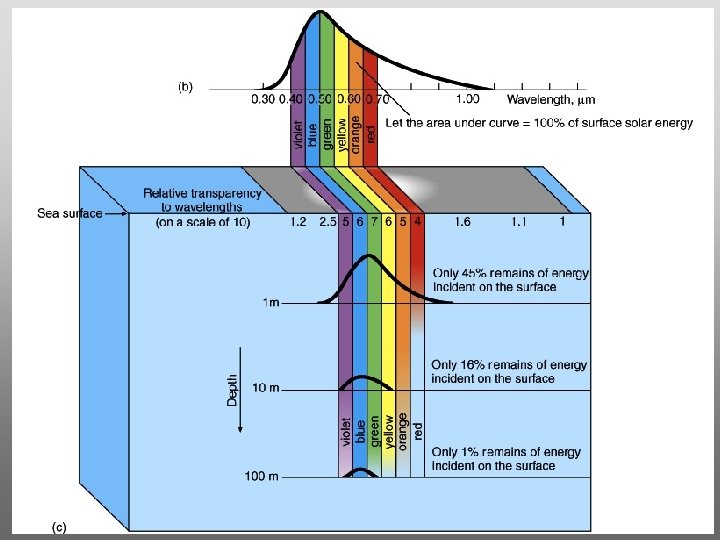
Evaporation from lakes, oceans, rivers, etc. occurs for temperatures lower than 100 o. C But it requires more energy to do so

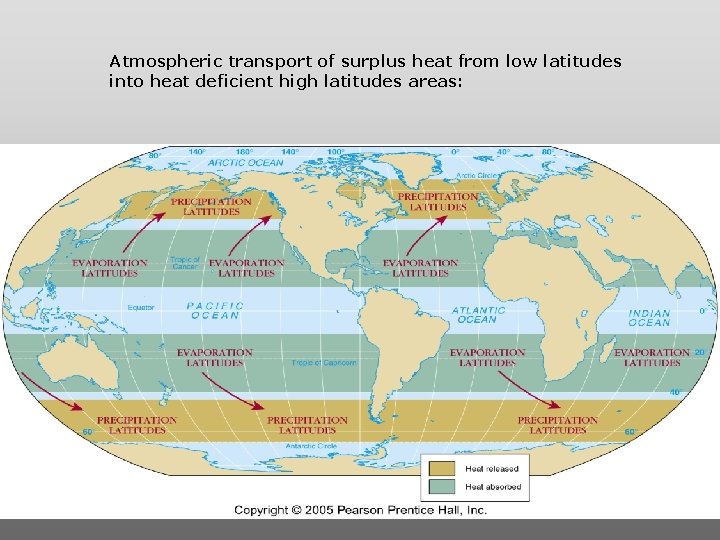
Atmospheric transport of surplus heat from low latitudes into heat deficient high latitudes areas:



