Water A Polar Molecule Moretz 2012 Biology Water

Water, A Polar Molecule Moretz, 2012 Biology

Water • Most abundant compound in living things. • Liquid on most of Earth’s surface. • Expands when it freezes.

Water • Water has a total charge of ZERO (10 neutrons + 10 protons + 10 electrons = no charge) • Water is POLAR = charges are not evenly distributed throughout molecule.
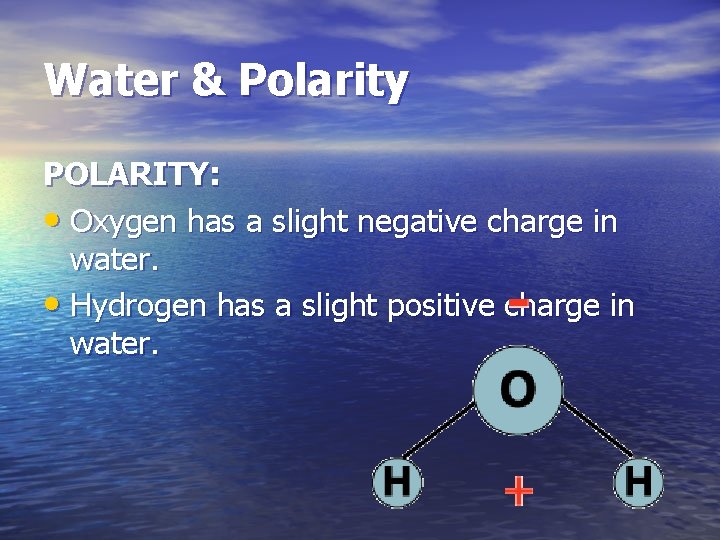
Water & Polarity POLARITY: • Oxygen has a slight negative charge in water. • Hydrogen has a slight positive charge in water.
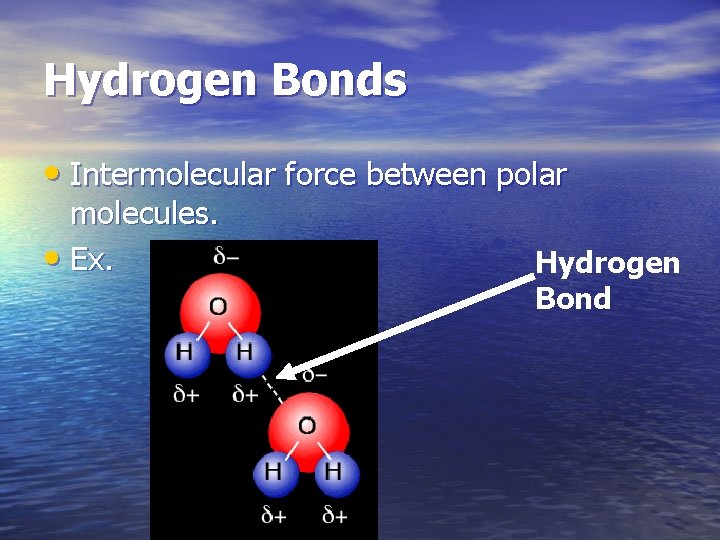
Hydrogen Bonds • Intermolecular force between polar molecules. • Ex. Hydrogen Bond

Hydrogen Bonds • Worksheet – Label the positively charged region in water and the negatively charged region in water. – Draw another water molecule near the original water molecule. Be sure to orient the water molecule correctly. – Add 3 more water molecules.
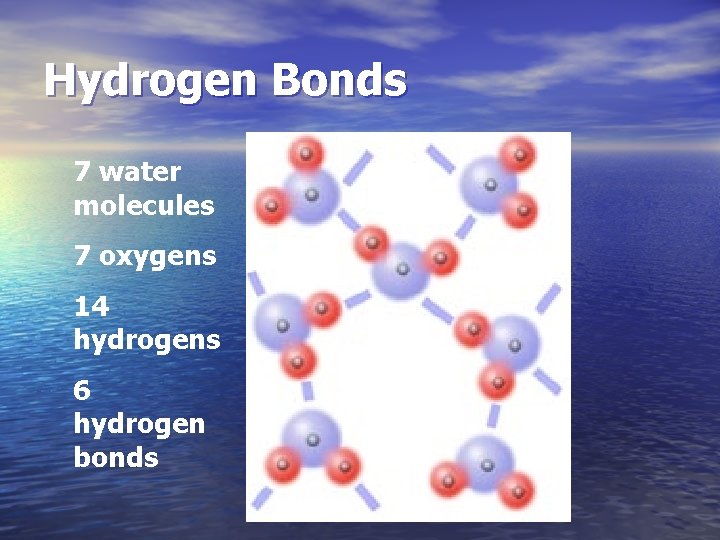
Hydrogen Bonds 7 water molecules 7 oxygens 14 hydrogens 6 hydrogen bonds
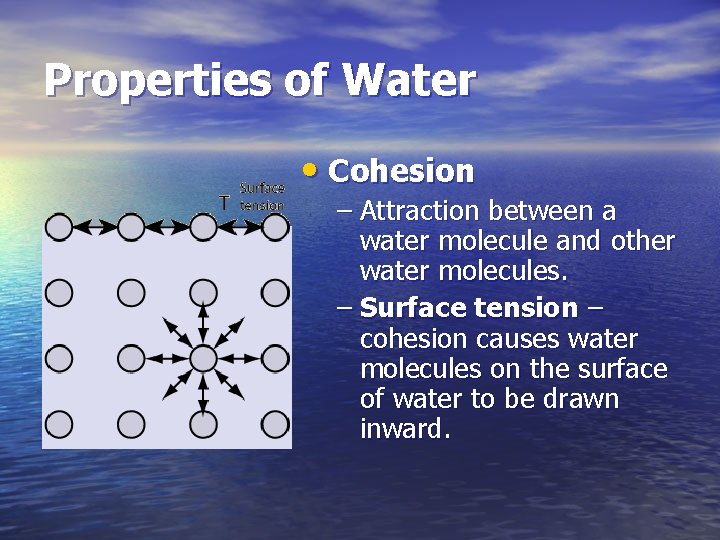
Properties of Water • Cohesion – Attraction between a water molecule and other water molecules. – Surface tension – cohesion causes water molecules on the surface of water to be drawn inward.

Cohesion

Surface Tension

Properties of Water • Adhesion – Attraction between water molecules and other substances. – Capillary action – adhesion causes water to rise up in a narrow tube.
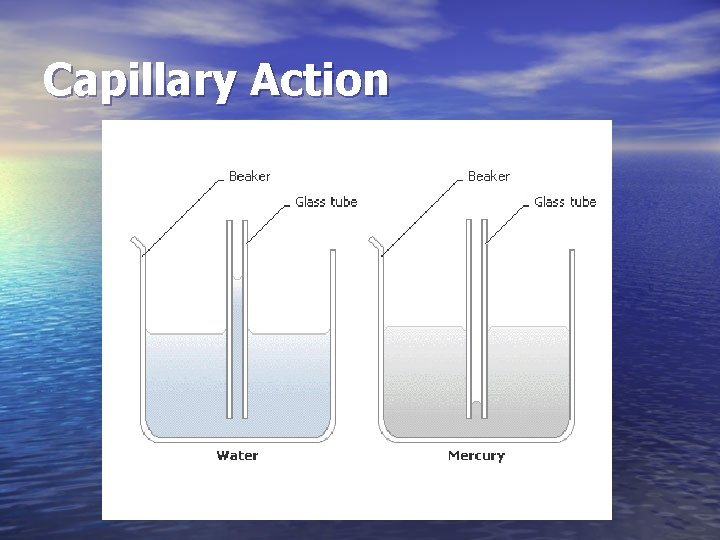
Capillary Action
- Slides: 12