WarmUp Which best represents the electron configuration for

Warm-Up • Which best represents the electron configuration for an atom of Mg+2? • A)1 s 22 p 63 s 2 • B) 1 s 21 p 62 s 22 p 63 s 2 • C) 1 s 22 p 6 • D) 1 s 22 p 63 s 23 p 64 s 24 d 6

Practice How many atoms are present in 23. 46 grams of Mn 2(SO 4)7? How many kilograms are in 3. 76 x 1024 particles of Fe(OH)3?

Practice • How many moles are present in 3. 45 x 1023 atoms of Ar? • How many atoms are present in 4. 56 moles of Fe? • Convert 6. 84 moles of Ca to particles.

Molar Volume • Molar Volume is often used to describe a gas • Gases can change volume based on temperature and pressure • STP-standard temperature and pressure

Molar Volume • Pressure: amount of force per unit area • STP- is a certain set of conditions • Standard Pressure is 101. 3 k. Pa or 1 atm • Standard Temperature is 0 C or 273 K

Molar Volume Continued • The volume of 1 mole at STP is 22. 4 L • Therefore, 1 mole (at STP) = 22. 4 L

Examples • 1) How many moles are in 45 L H 2 O at STP? • 2) How many liters are in 12. 3 moles of H 2 SO 4 at STP?

Classwork • 3) How many liters are in 1. 5 x 10 -10 moles of O 2 at STP? • 4) How many particles are present in 23. 4 L of a gas at STP?

Example • What volume is occupied by 90 g CO 2 at STP? • 1) Determine conversion factors • 2) Use factor label

Gas Density and Molar Mass • Recall, • 1 mole = 22. 4 L • 1 mole = gfm
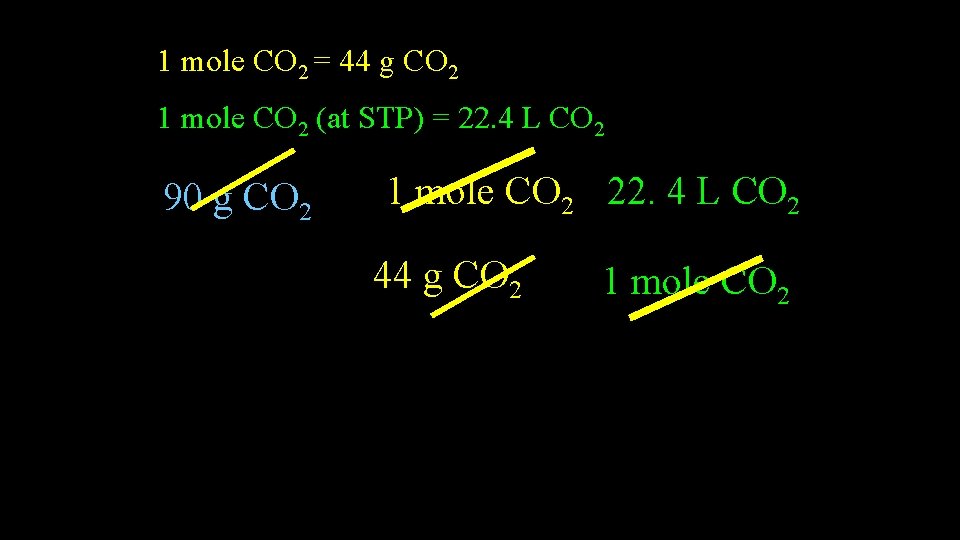
1 mole CO 2 = 44 g CO 2 1 mole CO 2 (at STP) = 22. 4 L CO 2 90 g CO 2 1 mole CO 2 22. 4 L CO 2 44 g CO 2 1 mole CO 2 = 45. 8 L CO 2

Review Questions • How many particles are in 54. 0 g NH 3? • How many grams are 3. 4 x 1024 particles of SO 3?

Practice • Determine the number of particles in 34. 5 g of H 2 SO 4. • How many grams are present in 2. 34 x 1025 particles of Br 2?

Practice • 1) How many atoms are in 19. 5 moles of Ca? • 2) How many grams are present in 0. 54 moles of K 2 S? • 3). How many grams of H 2 SO 4 are present in 9. 524 x 1025 atoms of H 2 SO 4?

Empirical Formula • The empirical formula {also called the simplest formula} gives the lowest whole number ratio of atoms of the elements in a compound

Empirical Formula • In an empirical formula, the number of atoms do not reduce any further • The subscript used must be expressed as a whole number (no decimal places)

Steps to Solve First determine the number of moles of each substance present • Convert the grams of each element to moles (using the gfm or molar mass) • 1) • 2) Then divide each number of moles present by the smallest number of moles • 3) (If Necessary) multiply the number of atoms by a number that will yield whole numbers
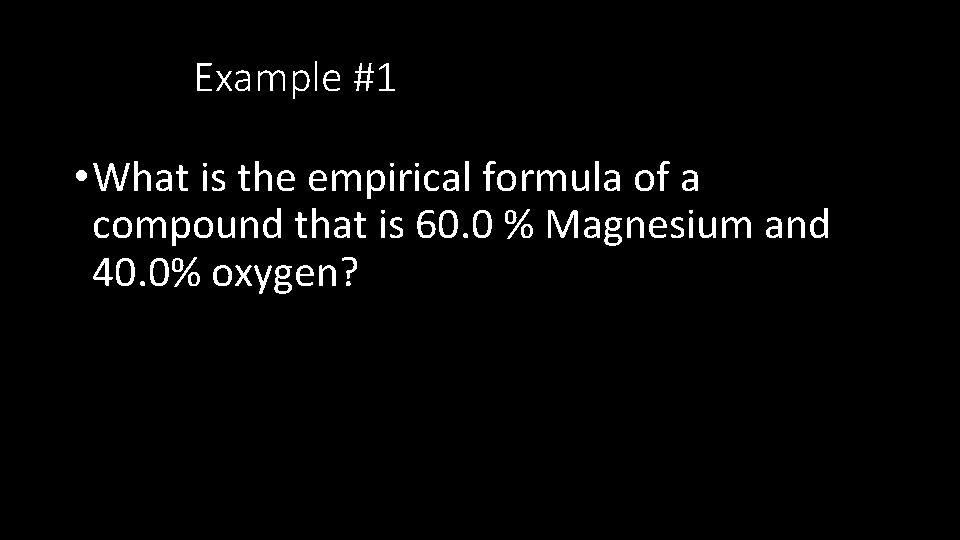
Example #1 • What is the empirical formula of a compound that is 60. 0 % Magnesium and 40. 0% oxygen?

Solution • Assume that 100 g of the compound are present • 60. 0% Magnesium= 60. 0 g Magnesium • 40. 0 % Oxygen = 40. 0 g Oxygen
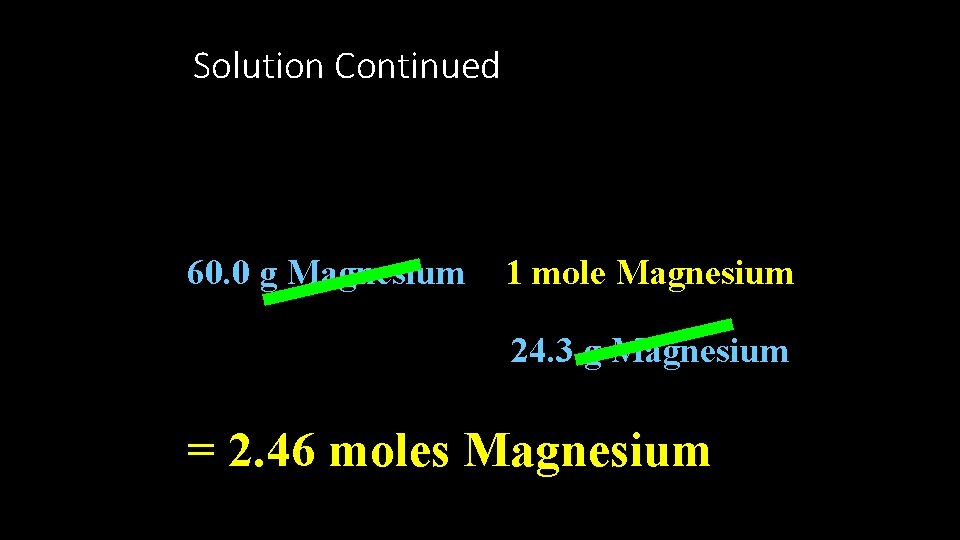
Solution Continued 60. 0 g Magnesium 1 mole Magnesium 24. 3 g Magnesium = 2. 46 moles Magnesium
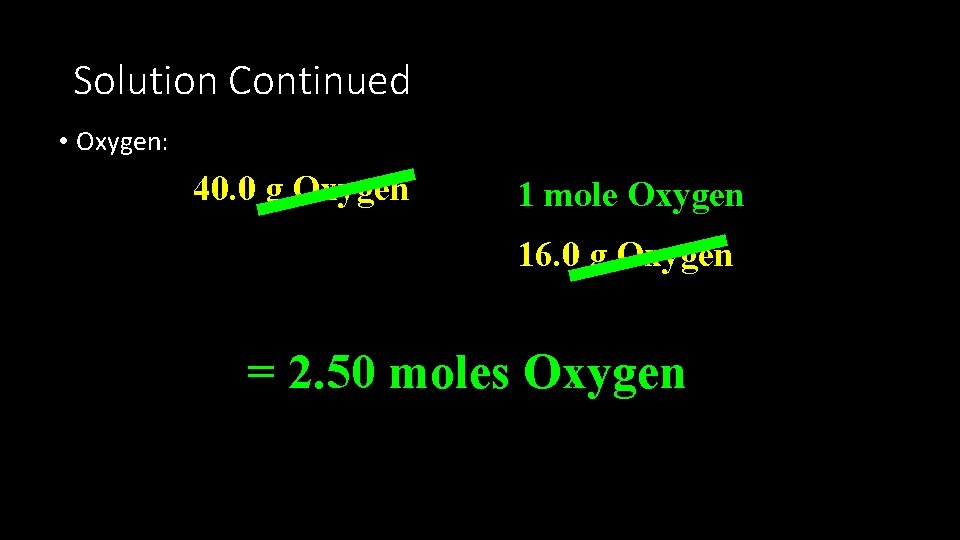
Solution Continued • Oxygen: 40. 0 g Oxygen 1 mole Oxygen 16. 0 g Oxygen = 2. 50 moles Oxygen

Solution Continued • 3) Now divide the number of moles for each element by the smallest number of moles. • 2. 46 moles Magnesium • 2. 50 moles Oxygen

Solution Continued • Magnesium has the smallest number of moles {2. 46 moles} • So divide the number of moles of Magnesium and Oxygen by 2. 46
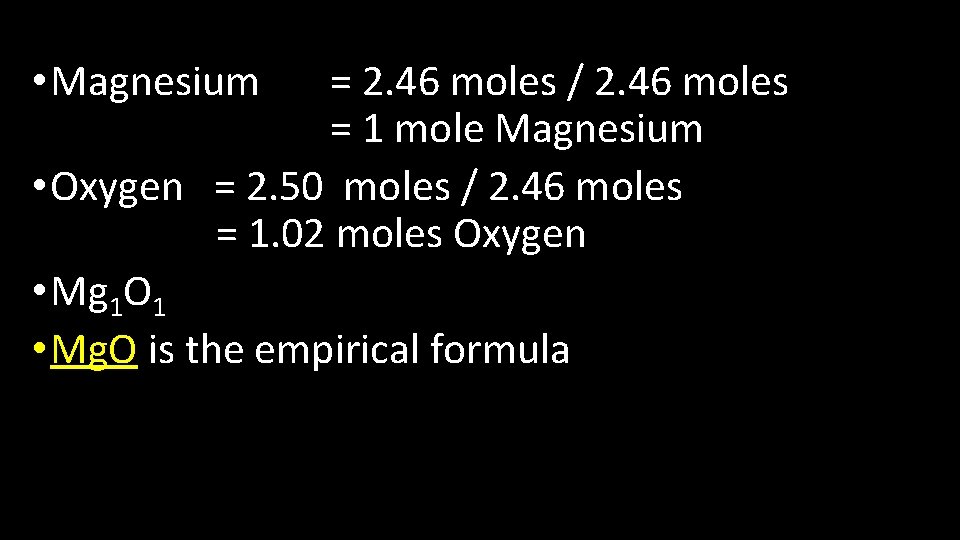
• Magnesium = 2. 46 moles / 2. 46 moles = 1 mole Magnesium • Oxygen = 2. 50 moles / 2. 46 moles = 1. 02 moles Oxygen • Mg 1 O 1 • Mg. O is the empirical formula
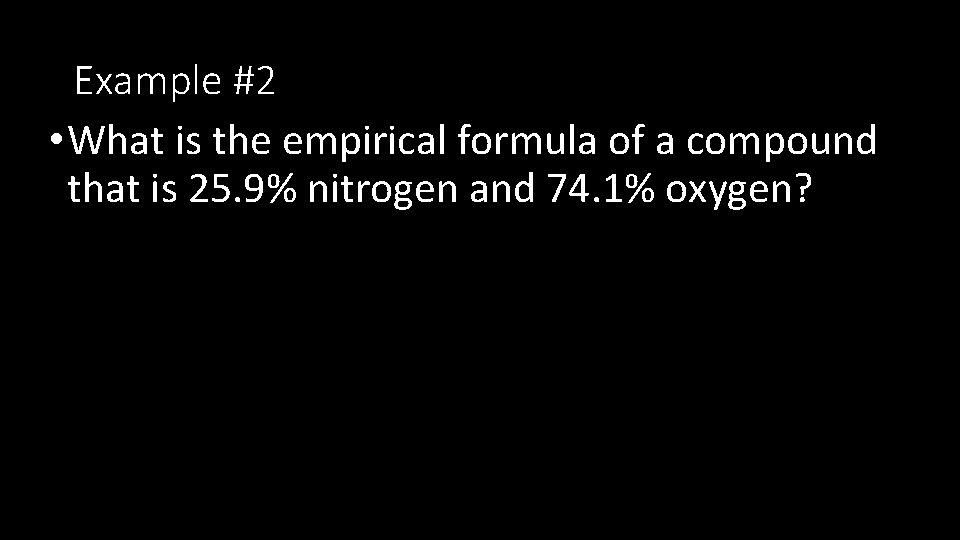
Example #2 • What is the empirical formula of a compound that is 25. 9% nitrogen and 74. 1% oxygen?

Solution • Assume that you have 100 g of the compound • 25. 9% Nitrogen = 25. 9 g Nitrogen • 74. 1 % Oxygen = 74. 1 g Oxygen
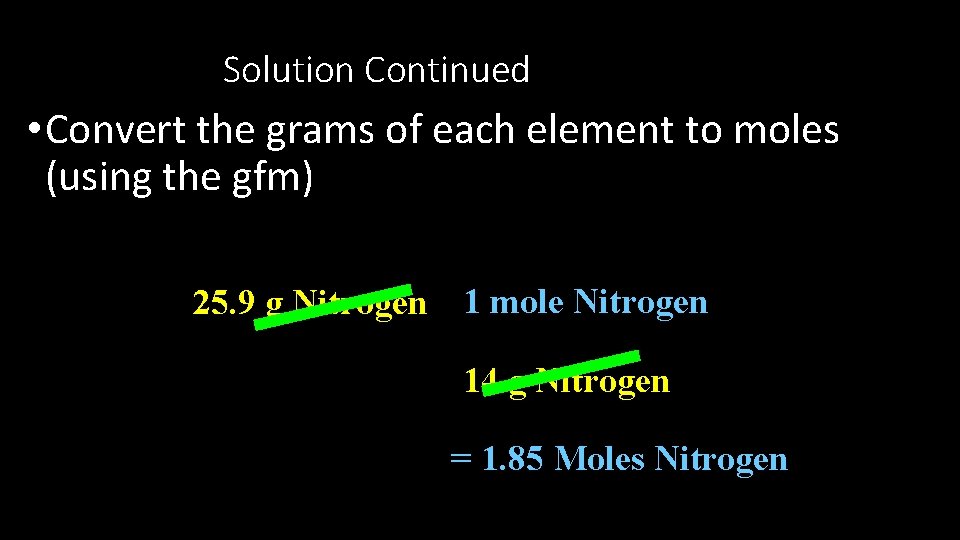
Solution Continued • Convert the grams of each element to moles (using the gfm) 25. 9 g Nitrogen 1 mole Nitrogen 14 g Nitrogen = 1. 85 Moles Nitrogen

Solution Continued • Oxygen 74. 1 g Oxygen 1 mole Oxygen 16 g Oxygen = 4. 63 Moles Oxygen

Solution Continued Now divide the number of moles for each element by the smallest number of moles. • 1. 85 moles Nitrogen • 4. 63 moles Oxygen • 3.

Solution Continued • Nitrogen has the smallest number of moles • So divide the number of moles of Nitrogen and Oxygen by 1. 85

Solution Continued • Nitrogen = 1. 85 moles / 1. 85 moles = 1 mole Nitrogen • Oxygen = 4. 63 moles / 1. 85 moles = 2. 50 moles Oxygen • N 1 O 2. 5

Solution Continued • Atoms can never appear in fractions or decimals • Thus, you must multiply the number of atoms by a number that will yield whole numbers • In N 1 O 2. 5, multiply the atoms by “ 2” • Thus N 2 O 5 is your final answer

Example # 3 • An 8. 20 g piece of magnesium combines completely with 5. 40 g of oxygen to form a compound. What is the empirical formula?

Additional Examples • Find the Empirical Formula of the following: • a. 71. 65% Cl, 24. 27% C, 4. 07% H • b. 43. 64% P, 56. 36% O

Practice • Find the empirical formula of a compound that contains: 27. 4% Na, 1. 19% H, 14. 3% C, and 57. 1% O
- Slides: 35