WarmUp How long can you go without water















- Slides: 29

Warm-Up How long can you go without water?

Questions? What is an atom? What is inside an atom?

Understanding Molecules and Atoms http: //www. youtube. com/watch? v=c. V 4 j. JZCIMPo http: //www. youtube. com/watch? v=Af. Xx. Zw. NLv. PA

Chemistry of Life Chapter 6 By Presenter. Media. com

Element • Made up of Atoms • Cannot be broken down into simpler substances Examples Carbon Hydrogen Oxygen

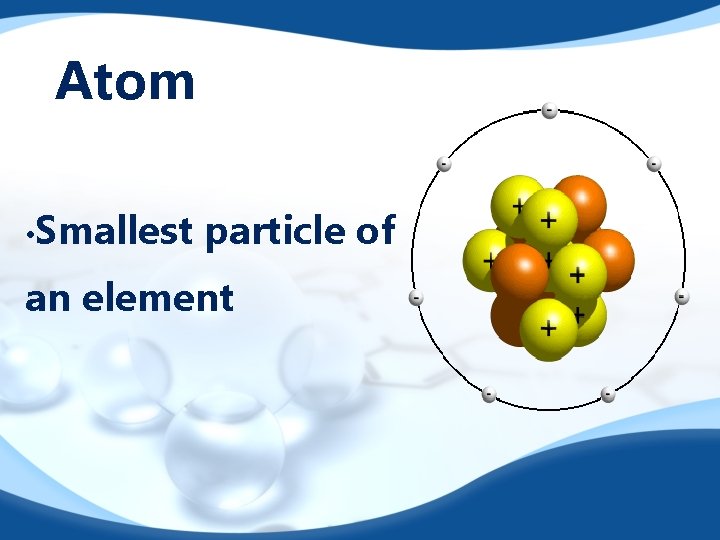
Atom • Smallest particle of an element

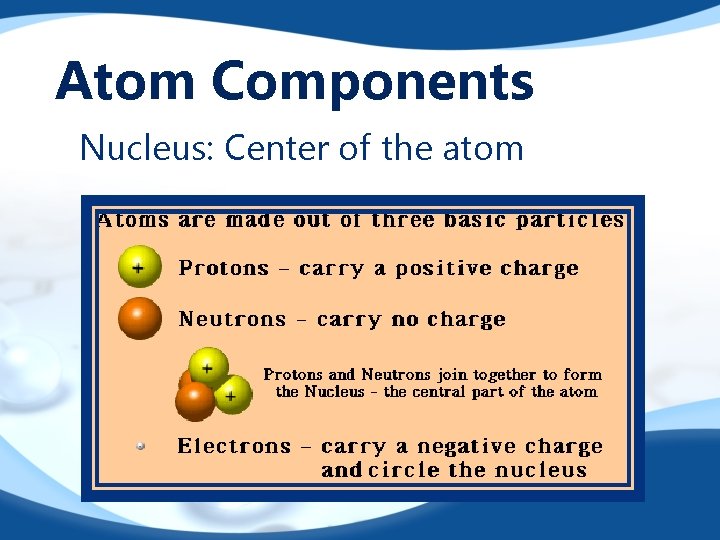
Atom Components Nucleus: Center of the atom


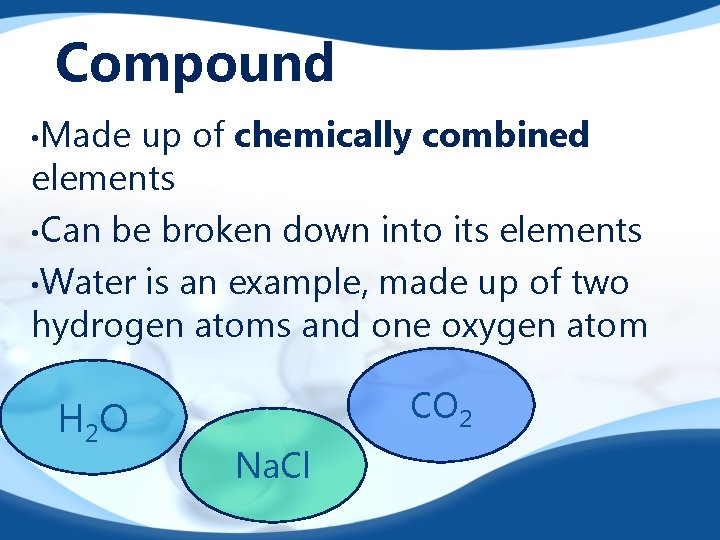
Compound • Made up of chemically combined elements • Can be broken down into its elements • Water is an example, made up of two hydrogen atoms and one oxygen atom H 2 O CO 2 Na. Cl

How Many Atoms… • Are in Glucose? • C 6 H 12 O 6 • 6 + 12 + 6 =24 • 24 atoms

Covalent Bond • Formed when two atoms share electrons • HINT: Covalent = Co Captain shares duties or Co-Pilot shares duties

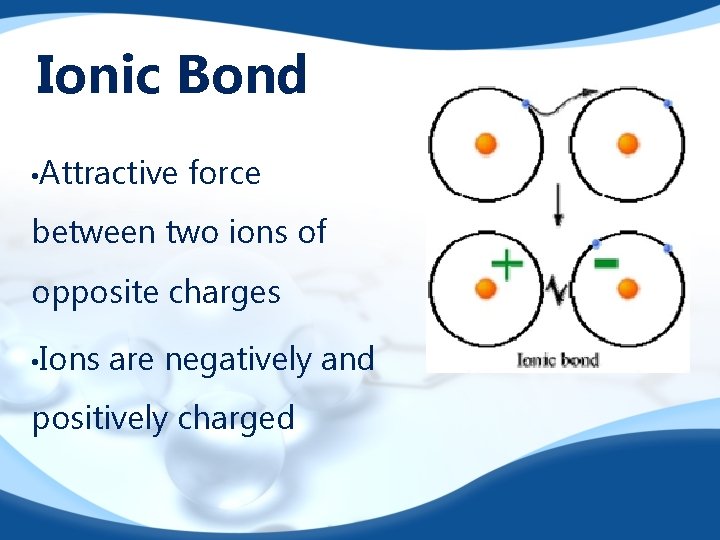
Ionic Bond • Attractive force between two ions of opposite charges • Ions are negatively and positively charged

Hydrogen Bond • Weak chemical bonds involving Hydrogen

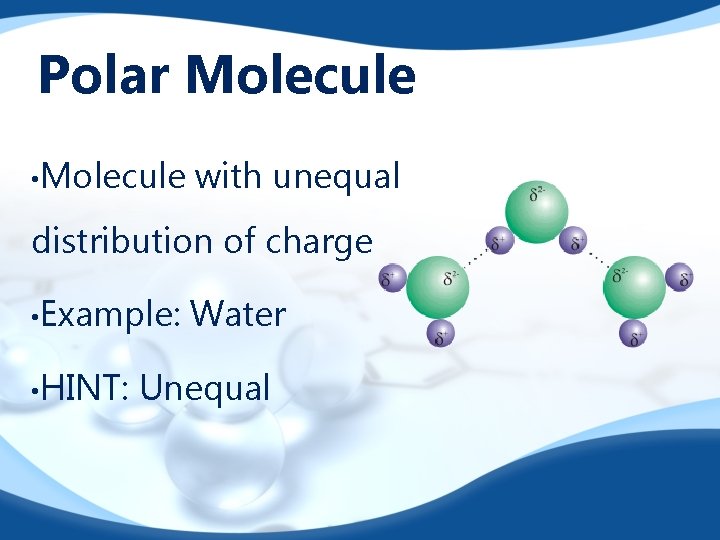
Polar Molecule • Molecule with unequal distribution of charge • Example: • HINT: Water Unequal

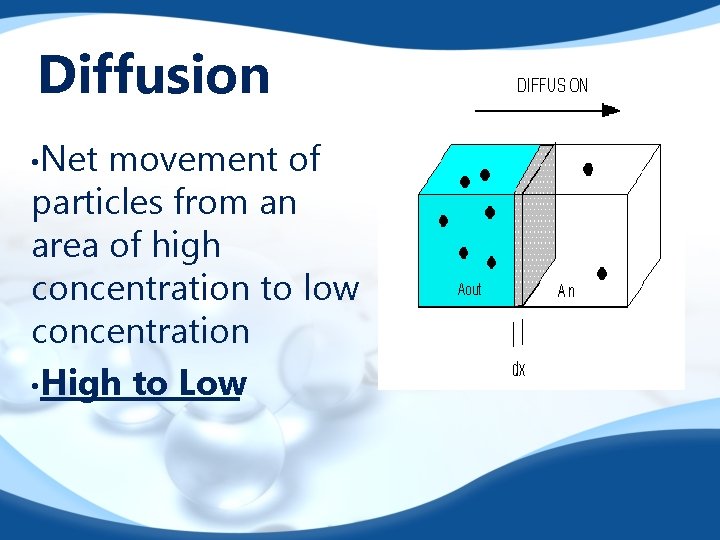
Diffusion • Net movement of particles from an area of high concentration to low concentration • High to Low

Dynamic Equilibrium • Continuous movement but no overall change in concentration • HINT: Think of a treadmill

Metabolism • All of the chemical reactions that take place within an organism. • Example: • Eating- the food we eat is turned into energy

p. H • Measures how acidic or basic • Acid: forms hydrogen ions in water H+ • Below 7 • Base: forms hydroxide ions OHin water • Above 7

Guess th e p. H

Warm-Up What do you call a substance made of 2 or more elements? a. b. c. a. Atoms protons compounds

Four Types of Organic Compounds that Make up Living Things • Carbohydrates • Lipids • Proteins • Nucleic Acids

Carbohydrates • Composed of carbon, hydrogen, & oxygen • CHO • Sugars and Starches (energy sources) • Starch comes from plants-mainly grains & potatoes 1. Monosaccharide – mono = 1, saccharide = sugar • Ex. Fructose, Glucose 2. Disaccharide • Ex. Sucrose – Table Sugar 3. Polysaccaride • Ex. Starch, glycogen, cellulose

Carbohydrates

Lipids • Composed of carbon, hydrogen, & oxygen • CHO • Energy Storage • Insoluble in water and Nonpolar. • Fats, Oils, Waxes and steroids

Proteins • Composed of carbon, hydrogen, oxygen, nitrogen CHON • Provide structure for tissues & organs • Abundant in muscle • Amino Acids – building blocks of protein 20 common amino acids • Peptide Bond – covalent bond between amino acids • Ex. Enzyme – changes the rate of chemical reaction

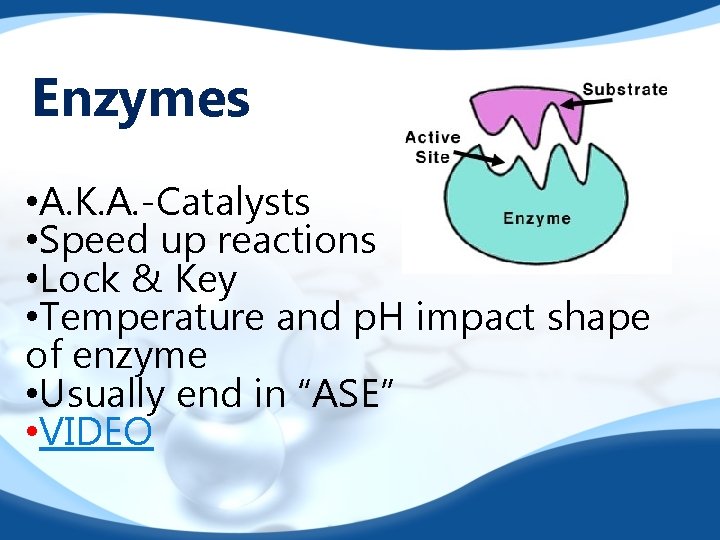
Enzymes • A. K. A. -Catalysts • Speed up reactions • Lock & Key • Temperature and p. H impact shape of enzyme • Usually end in “ASE” • VIDEO

Nucleic Acids • Polymers are made of subunits called nucleotides • Nucleotides: consist of carbon, hydrogen, oxygen, nitrogen and phosphorus • Example: DNA, RNA • Genetic material

Short review
