Warmup Find the electron orbital names and create

Warmup Find the electron orbital names and create a matching sketch for: Be Xe Also, figure out how many protons, electrons, and neutrons make up that atom in its neutral state.

Rydberg/Spectroscopy Experiment, Ionization Energies October 10, 2010

A Poem! Roses are 700± 30 nm Violets are 430± 30 nm I understand what Mr. Wurden said. Do you?
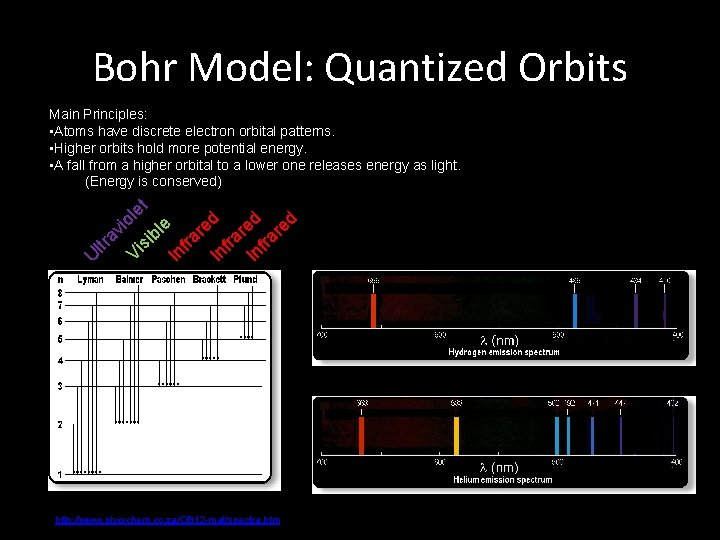
Bohr Model: Quantized Orbits Main Principles: • Atoms have discrete electron orbital patterns. • Higher orbits hold more potential energy. • A fall from a higher orbital to a lower one releases energy as light. (Energy is conserved) et l d d ed io le e e v r r r b tl ra isi nfra V I I I U http: //www. physchem. co. za/OB 12 -mat/spectra. htm

Relevant Equations Potential Energy PE = F * d Waves v=λ*f Quantization of Photons E=h*f Rydberg Formula 1/ λ = R Z 2 (n 1 -2 – n 2 -2) PE Potential Energy (J) F Force of attraction (N) d Distance (m) v velocity (3*108 m/s) λ wavelength (m) f frequency (Hz) E Energy (J) h Planck’s Constant (6. 63 * 10 -34 J*s) f frequency (Hz) λ wavelength (m) R Rydberg’s Constant (1. 1*10 -7 m-1) Z Atomic number n electron orbital number
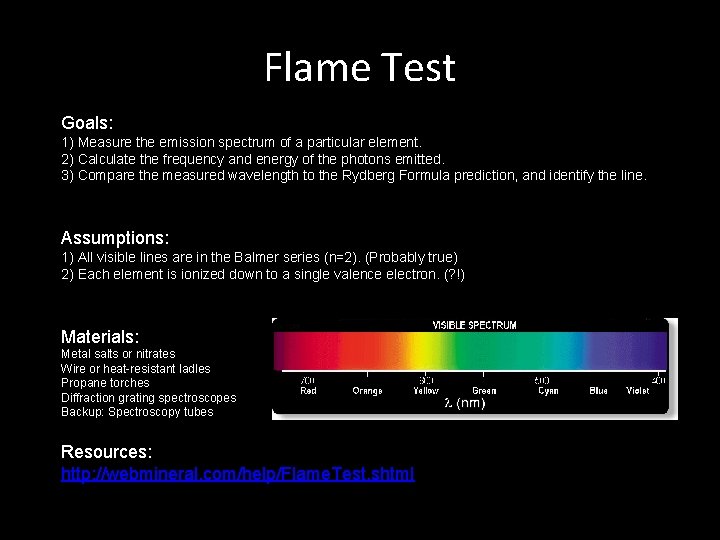
Flame Test Goals: 1) Measure the emission spectrum of a particular element. 2) Calculate the frequency and energy of the photons emitted. 3) Compare the measured wavelength to the Rydberg Formula prediction, and identify the line. Assumptions: 1) All visible lines are in the Balmer series (n=2). (Probably true) 2) Each element is ionized down to a single valence electron. (? !) Materials: Metal salts or nitrates Wire or heat-resistant ladles Propane torches Diffraction grating spectroscopes Backup: Spectroscopy tubes Resources: http: //webmineral. com/help/Flame. Test. shtml
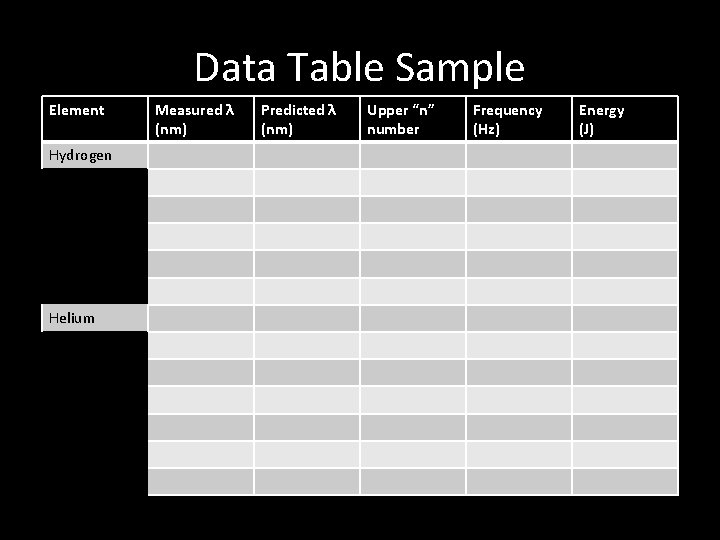
Data Table Sample Element Hydrogen Helium Measured λ (nm) Predicted λ (nm) Upper “n” number Frequency (Hz) Energy (J)
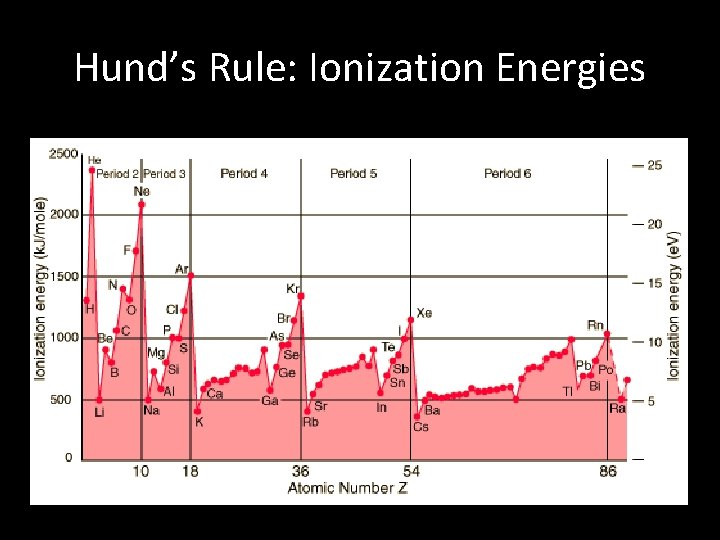
Hund’s Rule: Ionization Energies

Intro to Radioactive Decay October 10, 2010

Definitions • Isotope • Radioactive Decay • Alpha (α) Particle Emission (Helium nucleus) • Beta (β) Particle Emission (electron) • Gamma (γ) Radiation Emission (high-energy photon) • Half-Life (λ)
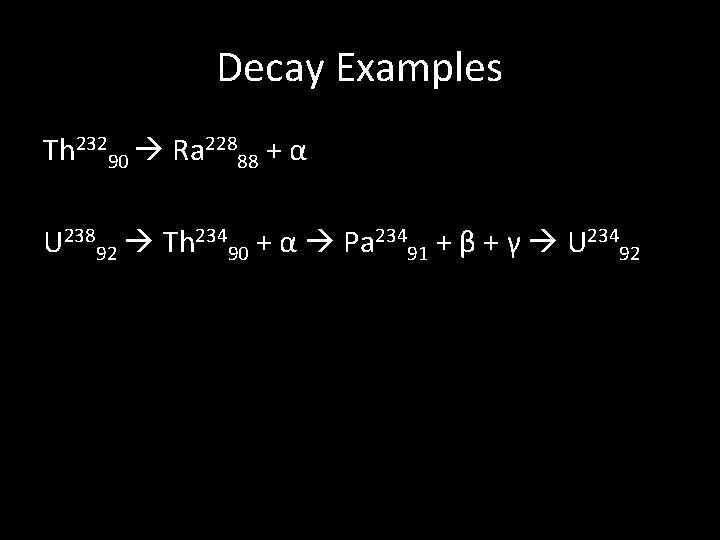
Decay Examples Th 23290 Ra 22888 + α U 23892 Th 23490 + α Pa 23491 + β + γ U 23492
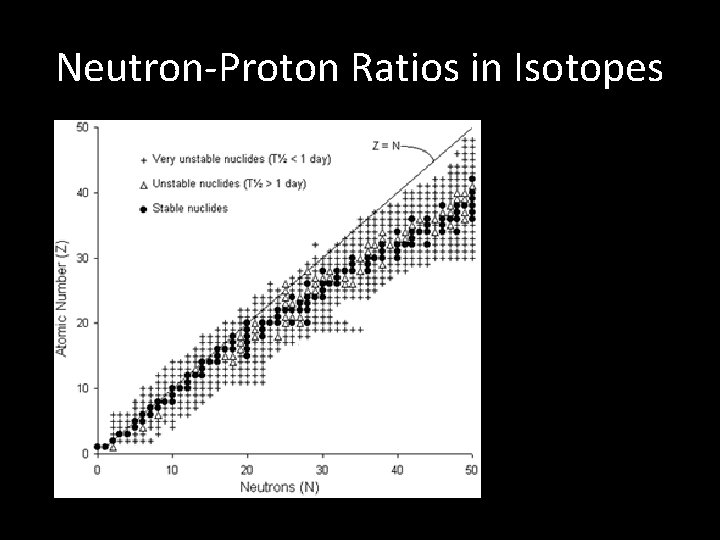
Neutron-Proton Ratios in Isotopes

Half-Life Calculations and Radioactive Dating

Fission vs. Fusion (Nuclear Reactions)
- Slides: 14