WARMUP 12215 1 Balance the following equations 2

WARM-UP 1/22/15 1. Balance the following equations: 2. a. __ K + __ F 2→ __ KF b. __ Na. Cl + __ H 2 SO 4 → __ Na. HSO 4 + __ HCl 2. Try to name each compound. It should be a review! Stamping in seven minutes.

1/22/15 Objective Recognize types of chemical reactions. 1/22/15 Agenda Quiz tomorrow – Balancing Equations Warm-Up Chemical Reactions

WARM-UP 1/22/15 1. Balance the following equations: 2. a. __ K + __ F 2→ __ KF b. __ Na. Cl + __ H 2 SO 4 → __ Na. HSO 4 + __ HCl 2. Try to name each compound. It should be a review!
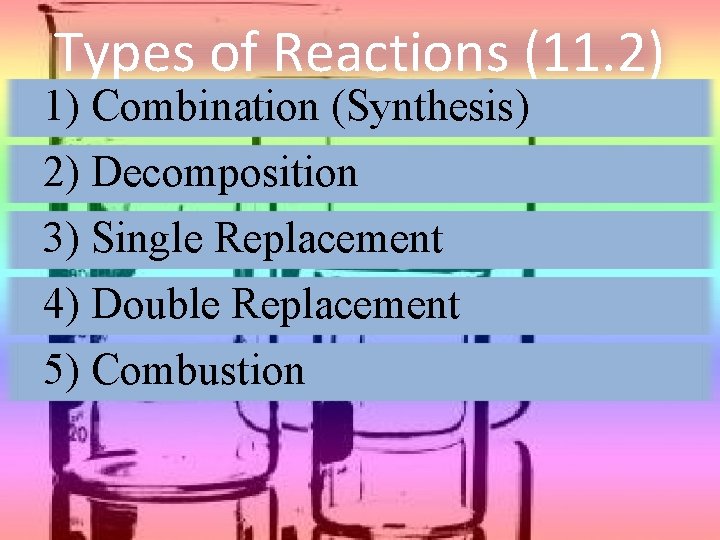
Types of Reactions (11. 2) 1) Combination (Synthesis) 2) Decomposition 3) Single Replacement 4) Double Replacement 5) Combustion
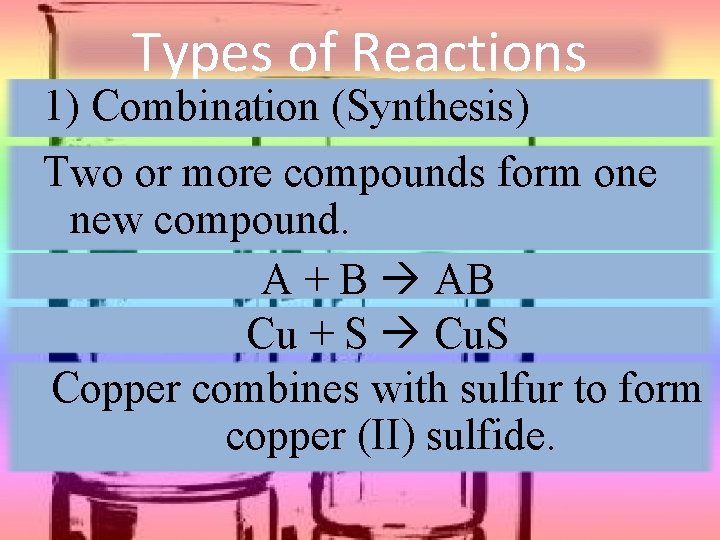
Types of Reactions 1) Combination (Synthesis) Two or more compounds form one new compound. A + B AB Cu + S Cu. S Copper combines with sulfur to form copper (II) sulfide.
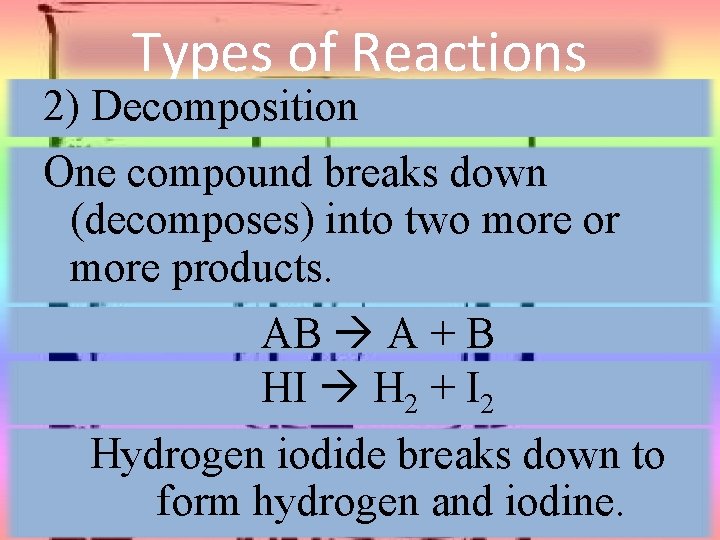
Types of Reactions 2) Decomposition One compound breaks down (decomposes) into two more or more products. AB A + B HI H 2 + I 2 Hydrogen iodide breaks down to form hydrogen and iodine.
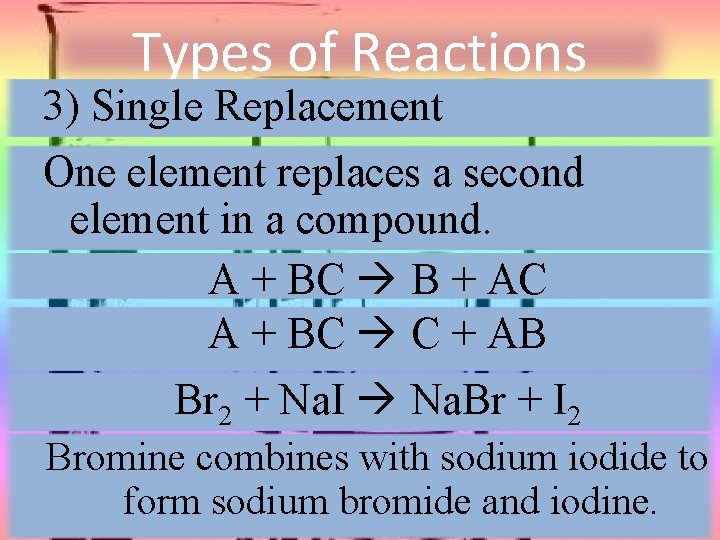
Types of Reactions 3) Single Replacement One element replaces a second element in a compound. A + BC B + AC A + BC C + AB Br 2 + Na. I Na. Br + I 2 Bromine combines with sodium iodide to form sodium bromide and iodine.
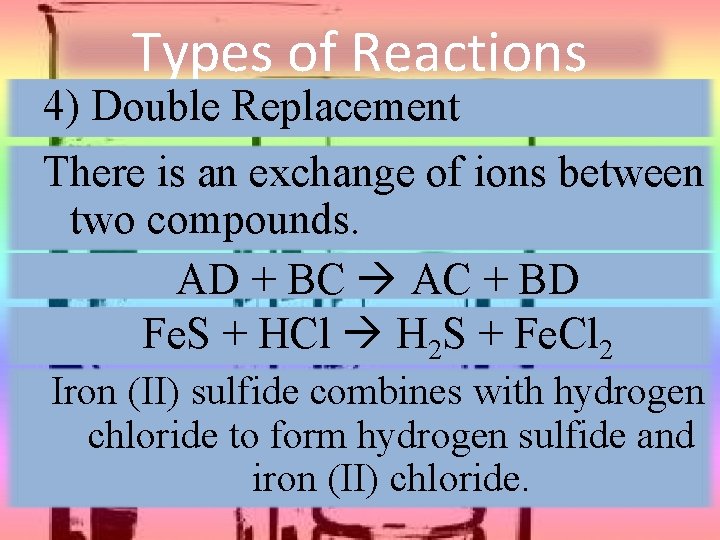
Types of Reactions 4) Double Replacement There is an exchange of ions between two compounds. AD + BC AC + BD Fe. S + HCl H 2 S + Fe. Cl 2 Iron (II) sulfide combines with hydrogen chloride to form hydrogen sulfide and iron (II) chloride.
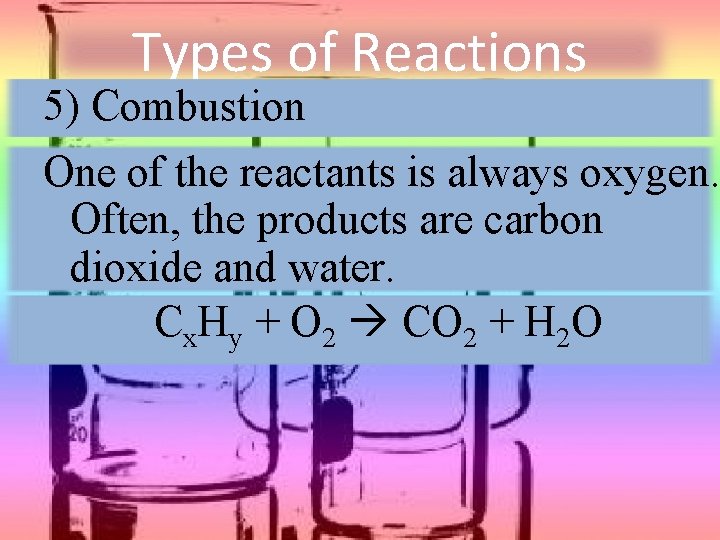
Types of Reactions 5) Combustion One of the reactants is always oxygen. Often, the products are carbon dioxide and water. Cx. Hy + O 2 CO 2 + H 2 O

You try! Classifying Reactions On either the Balancing Equations I or II, identify the different reactions. 1) Combination (Synthesis) 2) Decomposition 3) Single Replacement 4) Double Replacement 5) Combustion
Classifying Reactions 1) Combination (Synthesis) 2) Decomposition 3) Single Replacement 4) Double Replacement 5) Combustion

Tutoring Today (Thursday) Rm. 401

1/22/15 Homework 1) Collecting Balancing Equations I and II – balanced and properly labeled with chemical reaction. 2) Quiz tomorrow – Balancing Equations
- Slides: 13