Warmup 1 Give the names for the following




































- Slides: 57

Warm-up 1. Give the names for the following: CS 2, Cr. O, CH 4 and Mg. Cl 2 2. Give the formulas for the following: Nitric acid, aluminum oxide, copper (III) oxide, and dinitrogen pentoxide 3. What are the numbers on top and below an element on the periodic table? 4. Give a definition for the number below an element?

Holey Moley!

Ewwww…. Moles!

Moles…in Chemistry?

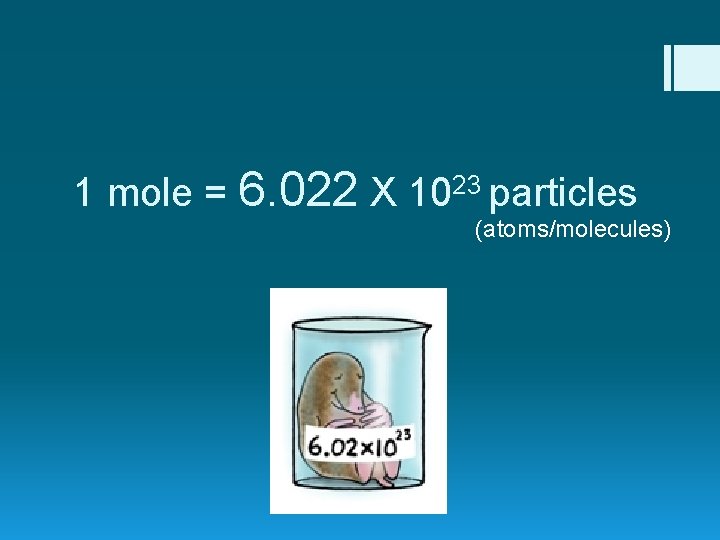
1 mole = 6. 022 X 1023 particles (atoms/molecules)

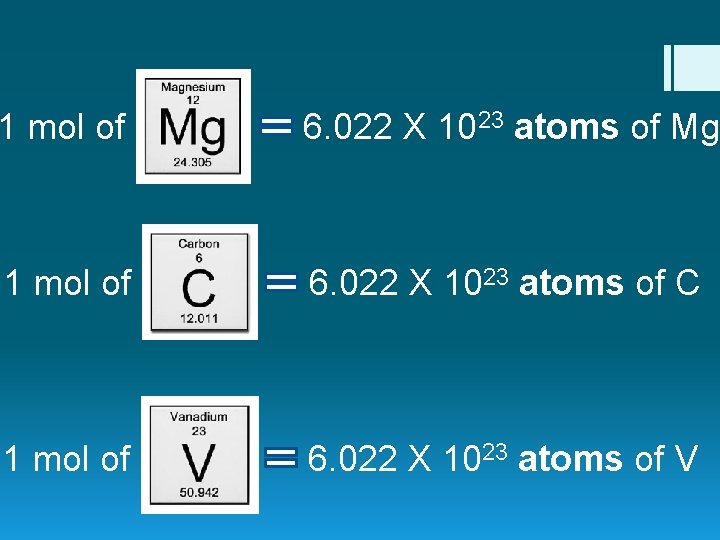
1 mol of 6. 022 X 1023 atoms of Mg 1 mol of 6. 022 X 1023 atoms of C 1 mol of 6. 022 X 1023 atoms of V

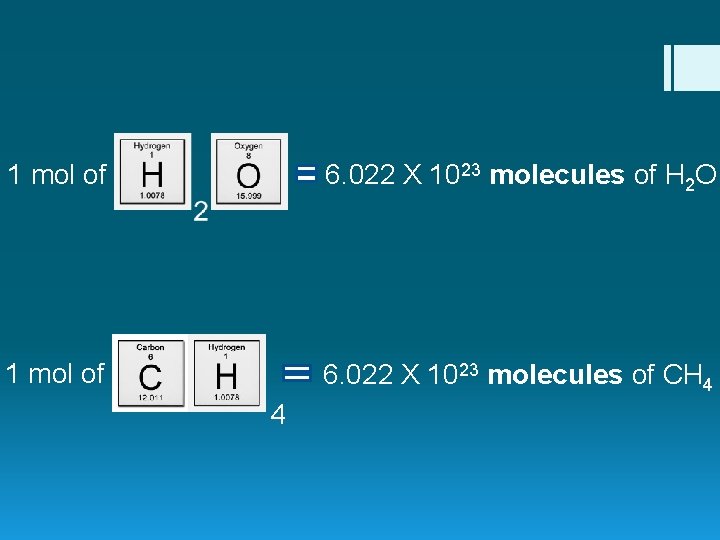
1 mol of 6. 022 X 1023 molecules of H 2 O 1 mol of 6. 022 X 1023 molecules of CH 4 4

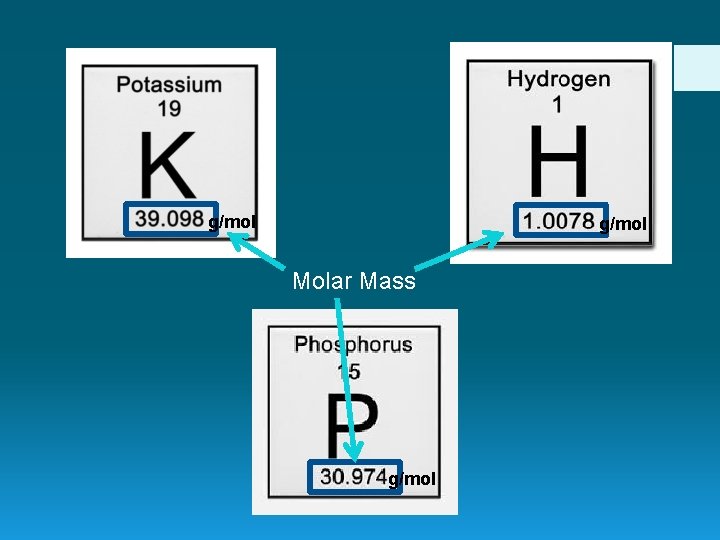
g/mol Molar Mass g/mol

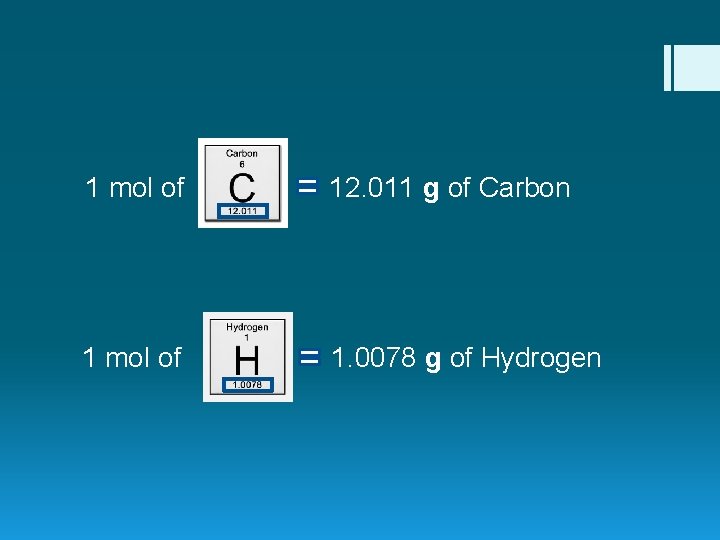
1 mol of 12. 011 g of Carbon 1 mol of 1. 0078 g of Hydrogen

Conversion Factors § 1 mole of substance = 6. 022 X 1023 atoms/molecules § 1 mole of substance = molar mass § Molar mass = 6. 022 X 1023 atoms/molecules

12 grams of K 2 S molecules

On Your Own! 1. 1000 grams of calcium is ______ moles of calcium 2. What is the mass of 3 moles of carbon disulfide? 3. How many moles of ammonia, NH 3, are in 51 grams of NH 3 4. A chemist needs 3 moles of aluminum oxide for an experiment. How many grams of aluminum oxide should she weigh out? 5. How many molecules of nitric acid are in 5 grams of nitric acid? 6. You guess that a solution of Cr. O contains 5. 0 X 1020 molecules. How many moles of Cr. O would be in this solution?

Warm-up 1. Give the name or the formula for the following: § § § K 2 CO 3 Nitric Acid Hydrochloric Acid Cu(OH)3 C 5 H 10 2. 1. 801 X 1024 atoms Cu = ? g Cu 3. What is the molar mass for C 11 H 12 O 11? 4. Define (in your words) the term “composition. ”

Chemistry’s Cornucopia

Cornucopia § Definition: an abundant supply of good things that are different.


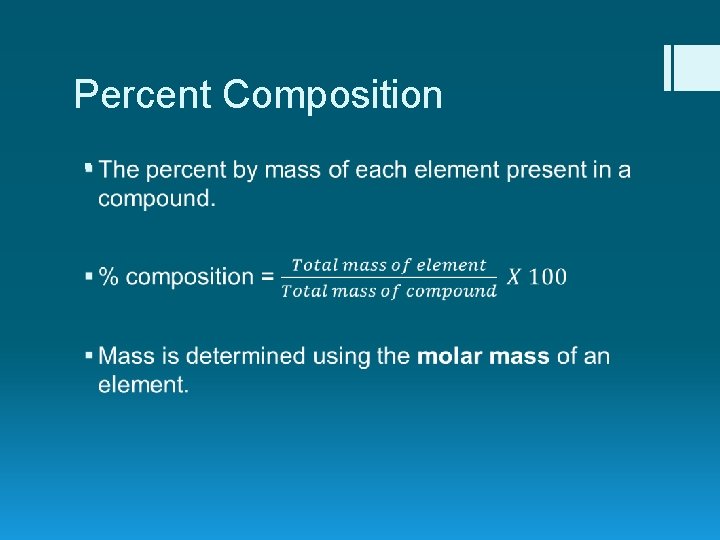
Percent Composition §


Mn(CHO 2)2 Mn O O C O H C H O

On Your Own! § Find the percent composition for each element of the following compounds: 1. 2. 3. 4. 5. C 12 H 22 O 11 Sulfuric Acid Potassium Permanganate Chromium (III) Sulfide Acetic Acid

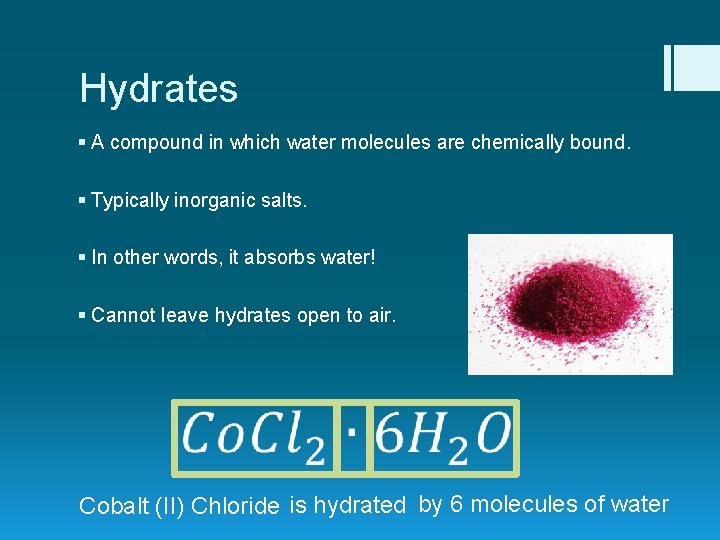
Hydrates § A compound in which water molecules are chemically bound. § Typically inorganic salts. § In other words, it absorbs water! § Cannot leave hydrates open to air. Cobalt (II) Chloride is hydrated by 6 molecules of water

Percent Composition of Hydrates § We use percent composition to determine the percent of water in a hydrate. § Important in determining unknown hydrates. § What is the percent water in Cobalt (II) Chloride Hexahydrate? https: //www. youtube. com/watch? v=Ou. F 4 hj. TFdsg

On Your Own! Determine the percent water for the following hydrates: §

“Step by Step” Calculating Empirical and Molecular Formulas

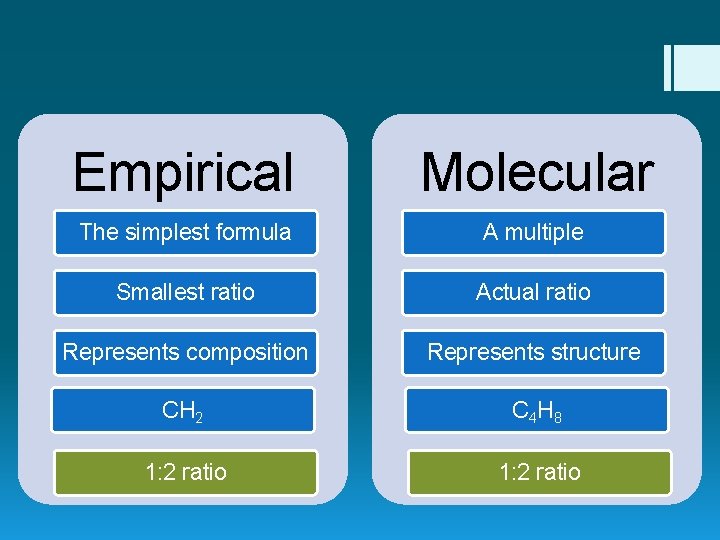
Empirical Formula § The simplest formula of a compound. § The smallest ratio of elements in a compound. § Represents the composition of elements in a compound. CH 2 1 C: 2 H “There is 1 carbon for every 2 hydrogens”

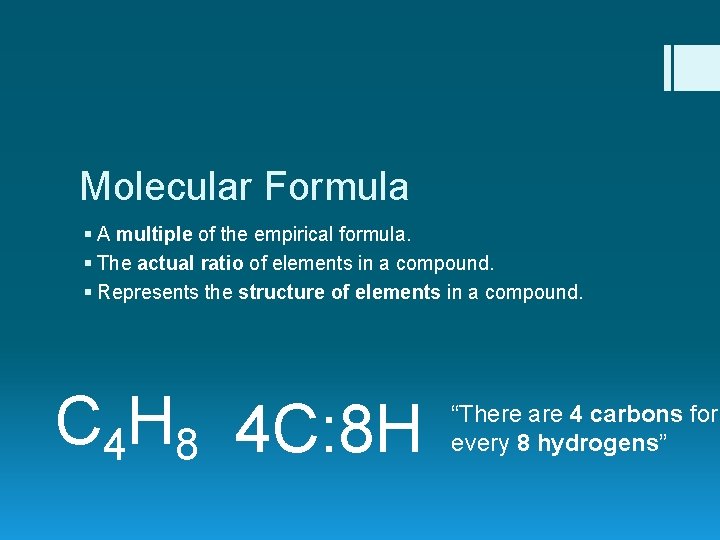
Molecular Formula § A multiple of the empirical formula. § The actual ratio of elements in a compound. § Represents the structure of elements in a compound. C 4 H 8 4 C: 8 H “There are 4 carbons for every 8 hydrogens”

Empirical Molecular The simplest formula A multiple Smallest ratio Actual ratio Represents composition Represents structure CH 2 C 4 H 8 1: 2 ratio

Empirical Formula Practice

“Step by Step” 1. 2. 3. 4. 5. Change percentage to grams assuming there are 100 g of the sample Divide by the molar mass of each element Divide the moles of each element by the lowest number of moles of all the elements Round to whole numbers to find ratios UNLESS there is. 5, then multiply each result by 2 for whole number ratios Write the formula!

Problem 1 The percent composition of a compound was found to be 63. 5% Silver, 8. 2% Nitrogen, and 28. 3% oxygen. Determine the compound’s empirical formula.

Problem 2 A 170. 00 g sample of an unidentified compound contains 29. 84 g of Sodium, 67. 49 g Chromium, and 72. 67 g Oxygen. What is the compounds empirical formula?

Problem 3 A 60. 00 g sample of an tetraethyl lead, a gasoline additive, contains 38. 43 g of Lead, 17. 83 g Carbon, and 3. 74 g Hydrogen. What is the compounds empirical formula?

Warm-up §

Molecular Formula Practice

“Step by Step” 1. Calculate empirical formula 2. Calculate the mass of the empirical formula 3. Divide the given mass by the empirical mass, this will give you an element multiplier 4. Multiply your chemical quantities by the multiplier 5. Rewrite molecular formula with new chemical values

Problem 1 § A compound contains 7. 19% phosphorus and 92. 81% bromine. If this compound has a molecular mass of 431 g/mol, what is the molecular formula?

Problem 2 § Naphthalene is a carbon and hydrogen containing compound often used in moth balls. The empirical formula is C 5 H 4 and its molar mass is 128. 16 g/mol. Find the molecular formula.

Investigating Chemical Reactions Evidence of Chemical Reactions

Prime Suspects §

Evidence #1 § Precipitate Formation § Highly predictable crime if we analyze solubility rules. § Soluble = dissolves in solution § Insoluble = forms a precipitate (solid) § Surveillance Footage: https: //www. youtube. com/watch? v=Qc 2 p. WUIz. P 2 k

Evidence #2 § Color Change § Intensity of color a result of a reactants being dilute or concentrated. § Surveillance Footage https: //www. youtube. com/watch? v=OKurc. Qt 3 ZOU

Evidence # 3 §

Common Tests for Chemical Reactions § Burning Splint – tests for oxygen, hydrogen, or carbon dioxide § https: //www. youtube. com/watch? v=Li. Av. Dpl 5 a. JA § Lime water – tests for carbon dioxide § https: //www. youtube. com/watch? v=QR 6 Gsyd. YUSI

Warm-up §

Types of Reactions

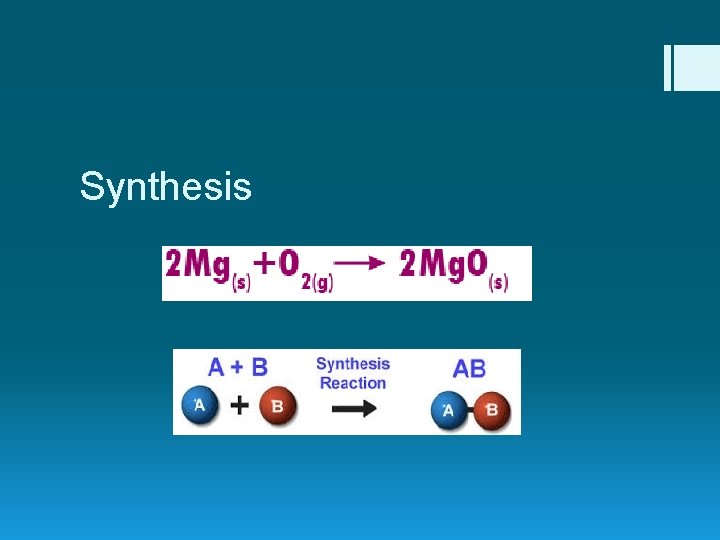
Synthesis

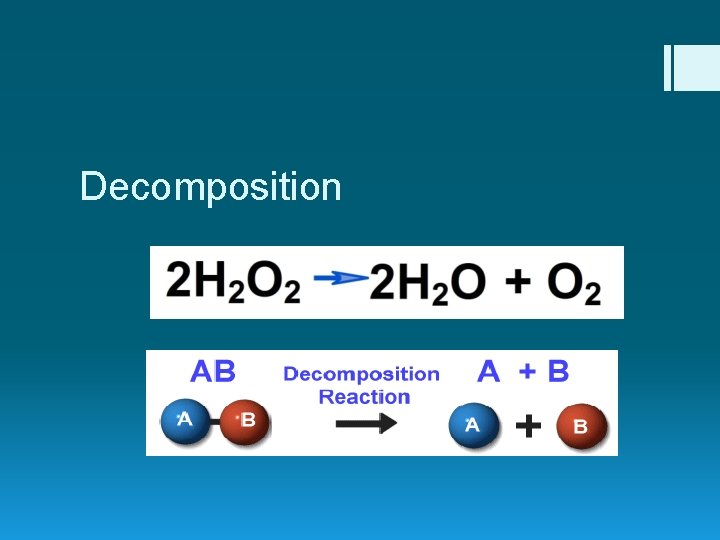
Decomposition

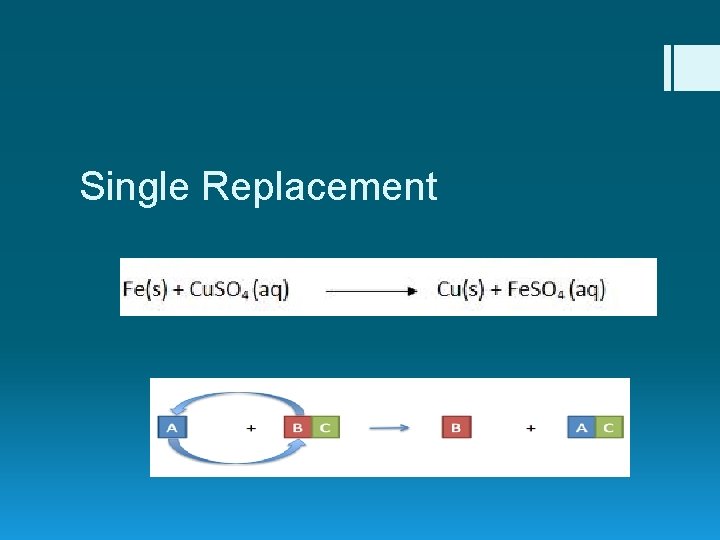
Single Replacement

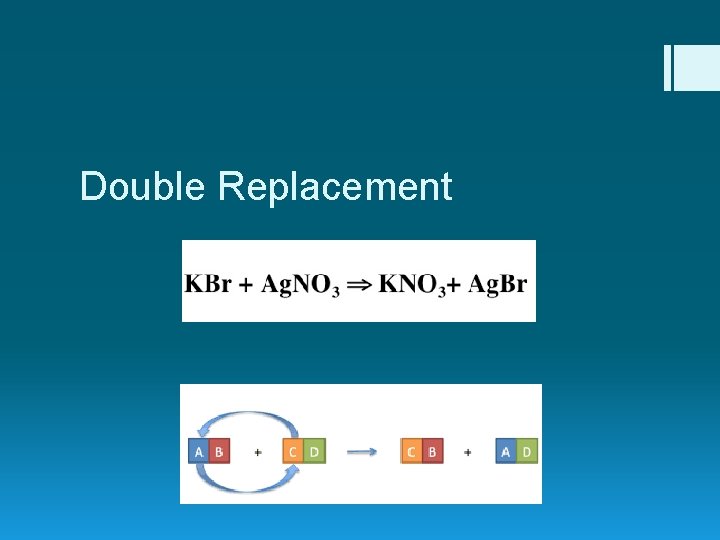
Double Replacement

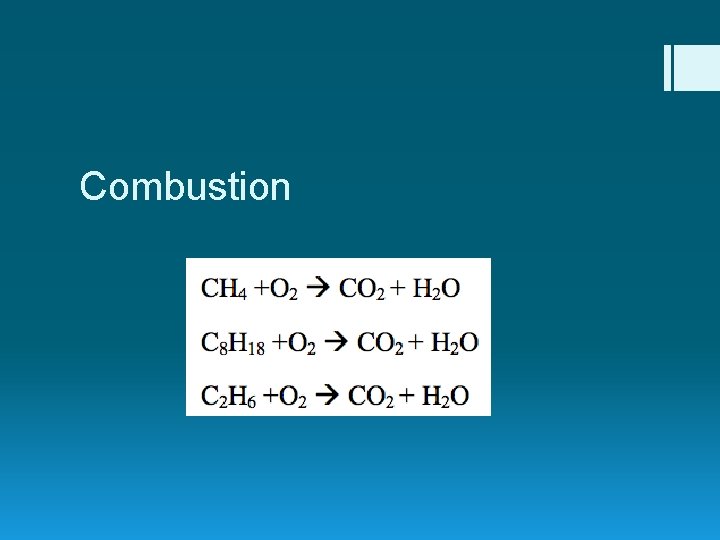
Combustion

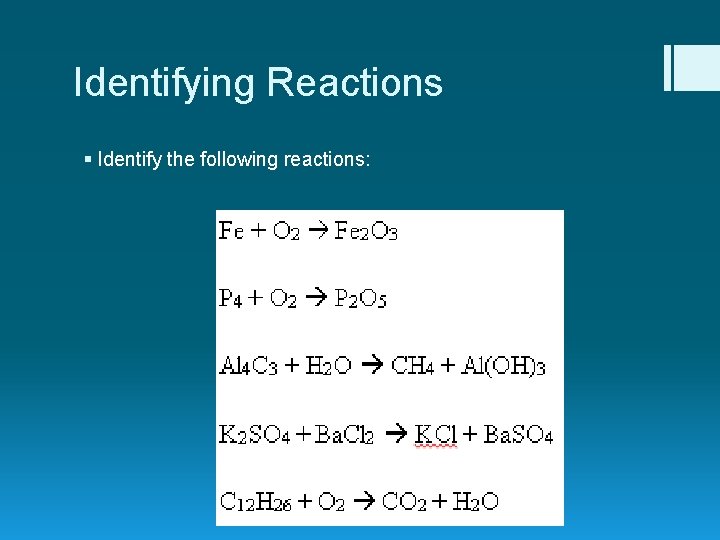
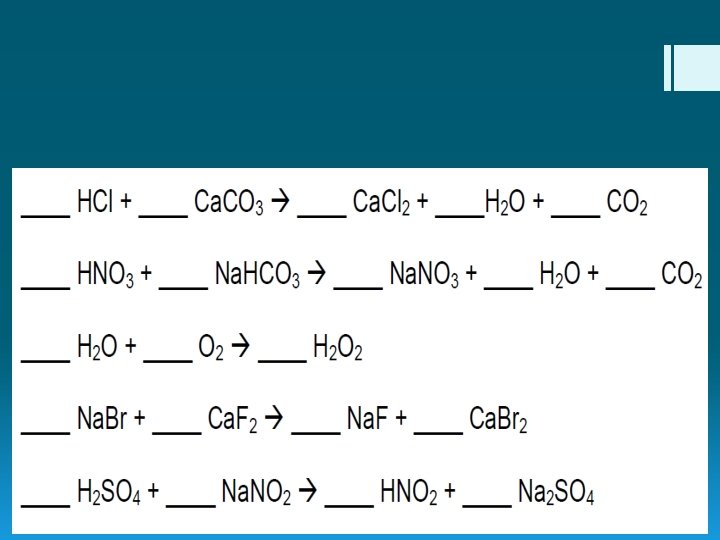
Identifying Reactions § Identify the following reactions:

Finding Your Balance Balancing Chemical Reactions

Balancing…What does it all mean?

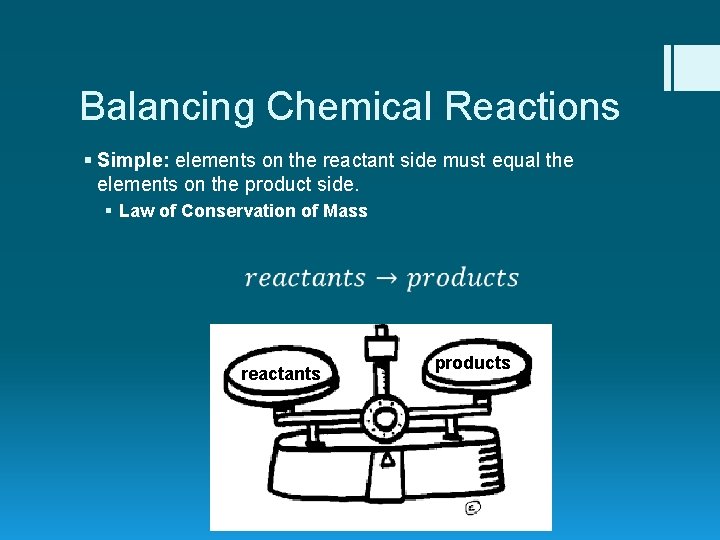
Balancing Chemical Reactions § Simple: elements on the reactant side must equal the elements on the product side. § Law of Conservation of Mass reactants products

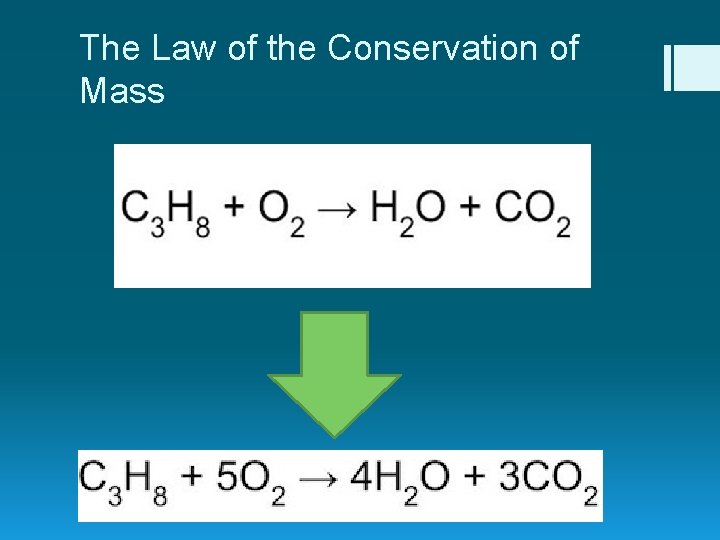
The Law of the Conservation of Mass

