WARM UPS 2016 2017 LAYOUT OF YOUR WARM









































































- Slides: 79

WARM UPS 2016 -2017

LAYOUT OF YOUR WARM UPS • • Number each Warm Up No more than two Warm-ups per page Include the problem and the answer Show all work for math problems

1 1. If you have been heating glassware, how should you pick it up? 2. What do you do if there is an accident in the lab? 3. Long hair should always be ______ in the laboratory. 4. Never leave a lit Bunsen burner _____. 5. _________ shoes are recommended in the lab.

2 1. When should you wear goggles in the lab? 2. If a chemical splashes on your skin, what should you do? 3. What should you do with unused chemicals? 4. How should you handle glass that you have been heating? 5. Should all chemicals be considered dangerous in the lab?

3 EXPLAIN WHAT WENT WRONG IN THESE LAB SITUATIONS 1. Diana and Mike were going to be late to their next class. After rushing to put away a few materials, they left the rest of the materials on the lab table. 3. The teacher was not in the room yet. Jake began weighing chemicals, touching them with his hands. His nose itched, so he rubbed it. 4. Cindy broke a test tube. Carefully she picked up pieces with one hand placed them in her other hand. Then she dumped the glass pieces into the wastebasket.

4 LABEL EACH AS A CHEMICAL OR PHYSICAL CHANGE 1. Liquid water freezing 2. Gasoline burning 3. Cooking an egg 4. If 2 clear liquids are mixed and small clumps fall to the bottom of the container. 5. A cube of orange colored sugar is dissolved into water resulting in an orange liquid.

LAB DIRECTIONS • Follow all directions • Finish on time – if you are done early, clean up and work on lab questions • Leave your lab area exactly as you found it! • Homework is on the front desk – just do the front page

5 Make 2 qualitative observations about the picture.

6 1. List the 3 of the 6 state changes. 2. How do you change matter from one state to another? 3. Is changing states a physical or chemical change?

7 1. A Bunsen burner mixes what two substances together? 2. What colors are a gas flame? 3. What laboratory tool is used to light a Bunsen burner? 4. Name 2 branches of Chemistry.

8 1. Sketch the heating curve of water. Label all the state changes. 2. Where are there two states coexisting?

9 1. What happens to temperature as a substance changes from one state to another? 2. Temperature is the measure of ________. 3. What is the difference between an element and a compound? 4. Compare homogeneous and heterogeneous mixtures.

10 1. What types of mixtures exhibit the Tyndall effect? 2. What are the two main types of heterogeneous mixtures? 3. What part of a solution is the solvent? 4. What is an alloy? 5. Why is water considered the “universal solvent”?

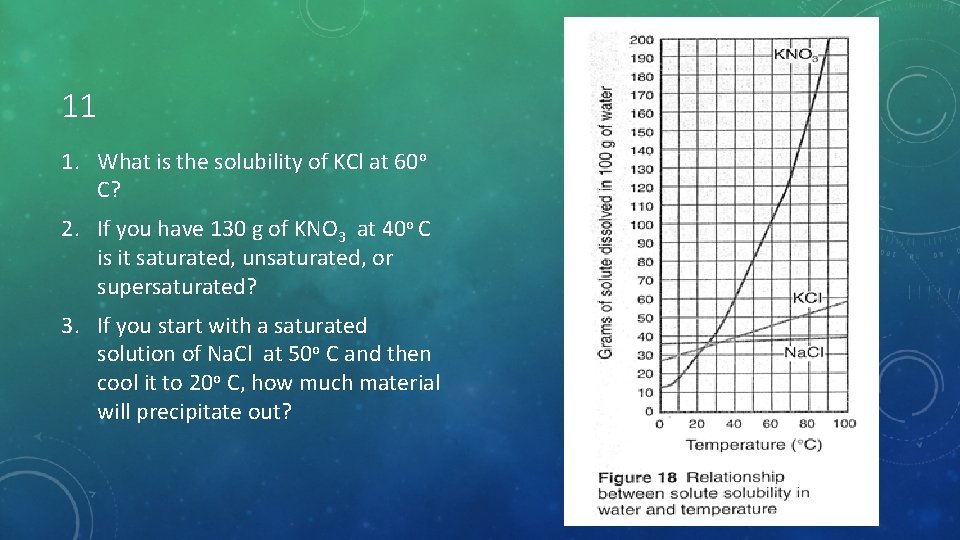
11 1. What is the solubility of KCl at 60 o C? 2. If you have 130 g of KNO 3 at 40 o C is it saturated, unsaturated, or supersaturated? 3. If you start with a saturated solution of Na. Cl at 50 o C and then cool it to 20 o C, how much material will precipitate out?

12 1. What is the main factor used to distinguish between the different kinds of mixtures? 2. Determine whether the following are chemical or physical changes: 1. Paper burning 2. Paper tearing 3. A metal and gas combining to form a white crystal 4. Water boiling 5. CO 2 subliming to form a gas 6. Sugar dissolving in water

13 1. List 5 physical and 5 chemical properties of water. 2. Using your qualitative observation skills, name 5 ways to determine the difference between a banana and a ham sandwich. Try to think of properties that can be used to tell any banana from any ham sandwich.

14 1. Classify each of the following as a(n): element, compound, homogeneous mixture, heterogeneous mixture 1. Oatmeal 2. Silicon dioxide 3. Lemonade 4. Chicken noodle soup 5. Xenon 6. Carbon monoxide 7. Salt water

WHEN YOU FINISH YOUR LAB • Turn off your Hot Plate (do NOT get rid of the Zn and Zn. Cl 2) • Clean up your lab space and finish your lab questions • Study for your test (retake tickets!)

15 1. Classify each of the following as a physical or chemical change 1. Salt dissolves in water 2. Grass grows 3. A nail rusts 4. A rock is split 2. Determine the state change 1. Water is placed out in winter (up north) 2. Ice forms on the outside a liquid nitrogen container 3. What is KMT?

16 HOW MANY SIGNIFICANT FIGURES IN EACH OF THE FOLLOWING? 1. 2. 3. 4. 5. 8. 01 80 8001 0. 0081

17 • How many significant figures do the following number have: • 0. 004 • 3. 854 • 5004 • 56. 02 • 58. 00 • 1000

18 PERFORM THE OPERATION AND ANSWER WITH THE CORRECT SIGNIFICANT DIGITS 1. 4. 328 + 234. 8 = 2. 4. 328 x 234. 8 =

19 USE DIMENSIONAL ANALYSIS TO SOLVE 1. 14 centuries to years 2. $3. 50 to quarters 3. 900 cm to meters

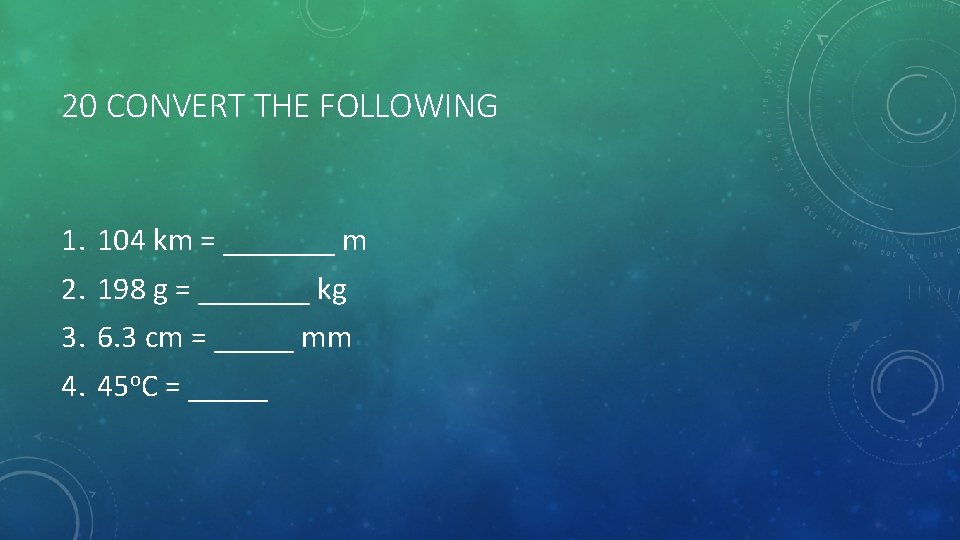
20 CONVERT THE FOLLOWING 1. 2. 3. 4. 104 km = _______ m 198 g = _______ kg 6. 3 cm = _____ mm 45 o. C = _____

21 1. How many grams in 1 mol of the following: 1. Ga 2. Pt 3. Ba 2. How many moles in the following 1. 102. 91 g of Rh 2. 265. 82 g Cs 3. 8. 00 g O

22 1. 6. 95 x 1023 atoms Zn g 2. 2. 99 moles Li g 3. 8. 94 moles N atoms

23 1. 6. 34 mol Sr atoms 2. 9. 04 g H atoms 3. What is the density of an object that has a mass of 8. 56 g and a volume of 5. 234 m. L? 4. What is the mass of an object that has a density of 56. 23 g/cm 3 and a volume of 3. 25 cm 3?

24 1. How many significant figures? 90. 001 2. Round to 3 significant figures – 65434. 23 3. Calculate. Show the intermediate steps. (7. 843 x 6. 34) + 43. 23 = 4. Convert using dimensional analysis: 143. 2 m in 5. Convert 364 K = o. C 6. A piece of wood that measures 3. 0 cm by 6. 0 cm by 4. 0 cm has a mass of 80. 0 grams. What is the density of the wood? Would the piece of wood float in water? (volume = L x W x H)

WHEN YOU ARE FINISHED WITH YOUR QUIZ • Make sure you have included your version on your paper! • Turn in your quiz • Get a Test Review and a Lab paper • Begin the lab • If you have time, find the density of multiple samples. Please return each sample you have measured to the box in the front of the room as you are finished.

25 1. How many grams does 0. 500 moles of Br weigh? 2. If a doctor finds that his patient has a mass of 65 kilograms, how heavy is the patient in grams? 3. How many weeks are there in 34 hours? 4. How hot is 4500 C in Kelvin? 5. 349 cm + 1. 10 cm + 100 cm =

26 1. Who was the first person to describe “atoms”? 2. Name 3 more scientists who added to Atomic Theory. 3. Describe “plum pudding” model of the atom and how it was developed. 4. Who discovered the nucleus? What experiment did he use?

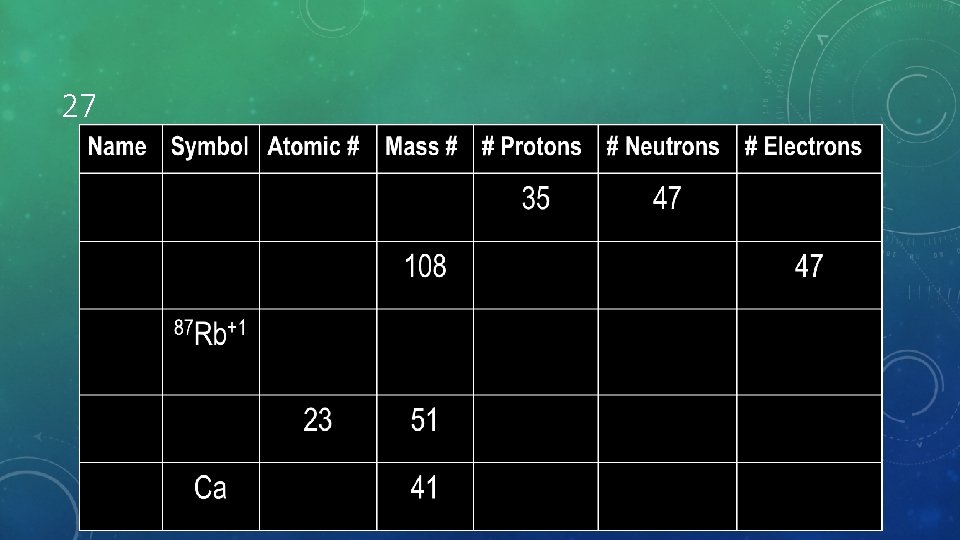
27

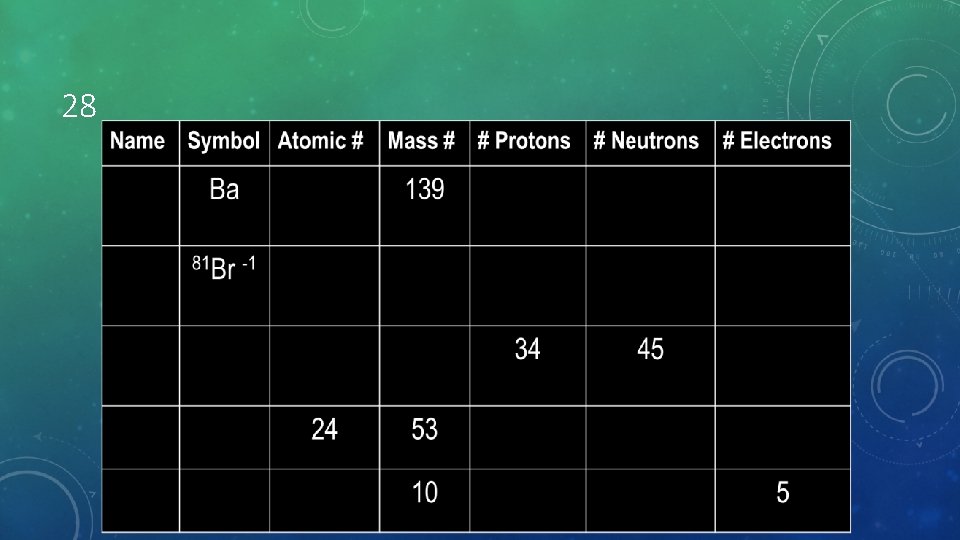
28

29 1. A radio station broadcasts at a frequency of 5. 90 x 105 Hz. What is the wavelength of the radio waves? 2. Electrons have properties of both _____ and _____. 3. Wavelength and frequency are _____ related.

30 1. What is average atomic mass of Lithium if 7. 42% exists as 6 Li (6. 015 amu) and 92. 58% exists as 7 Li (7. 016 amu)? 2. _____ was the first person to suggest the existence of atmos. 3. The law of conservation of mass states that mass is not ____ nor ____ in chemical reactions. 4. There are ____ postulates to Dalton’s Atomic Theory. 5. ____ discovered the electron. 6. _____discovered the nucleus.

31 1. Magnesium occurs in nature in three isotopic forms: • 24 Mg (78. 70% abundance) 23. 985 amu • 26 Mg (11. 17% abundance) 25. 983 amu • 25 Mg (10. 13% abundance) 24. 986 amu Calculate the atomic mass of Magnesium from this data

32 1. Calculate the energy of light that has a frequency of 1. 5 x 1015 Hz. 2. A photon has the energy of 1. 78 x 10 -27 J, calculate its frequency.

33 1. What is the Orbital Configuration of: 1. C 2. P 3. Zn 4. As

34 1. What is the Electron Configuration of: 1. C 2. P 3. Zn 4. As

35 1. What is the Nobel Gas Configuration of: 1. C 2. P 3. Zn 4. As

36 1. The _______ number gives the main energy level. 2. The ______ number gives the shape of the sublevel. 3. The ____ number gives the orientation around the nucleus. 4. The _____ number gives the direction an electron is traveling. 5. Complete the electron configuration of S using noble gas notation.

37 1. What is the difference between Meneleev’s Periodic Table and modern Periodic Table? 2. What is the Octet rule and what are valence electrons? 3. Name the major groups of the PT 4. Give all three types of electron configurations for Cl.

38 1. Explain Hund’s rule. 2. Explain the Aufbau principle. 3. Explain the Pauli Exclusion principle. 4. How many electrons are in an atom with ending electron configuration 4 d 6? 5. What element has an electron configuration that ends in 3 p 5?

39 1. _____ is the father of the modern periodic table. 2. The original periodic table was arranged by atomic ____. 3. What parts of Dalton’s theory were proven false? 4. How many p+, n 0, and e- in 34 S-2?

40 1. Put the following elements in order from least to greatest atomic radius: V, Ga, Br, K 1. Why is water considered the “universal solvent”? 2. Convert 42. 2 km to miles if 2. 54 cm=1 in and 5280 ft=1 mi 3. What is the electron configuration for B?

41 1. How many electrons would each of the following have to gain or lose to become stable while forming an ion? What ion would they form? 1. Na 2. Mg 3. Al 4. Si 5. P 6. Cl 7. Ar

42 1. 2. 3. 4. 5. What kind of an ion is K+? How many electrons has Br- gained or lost? What is the noble gas configuration of O-2? How many valence electrons does Nitrogen have? What type of bond does Na – Cl form?

43 1. What is the most common ion formed by the following: 1. Ca 2. N 3. Ga 4. I 2. What is the relationship between length of the bond and bond energy? 3. How are ionic bonds formed? 4. How are covalent bonds formed?

44 1. Name the shape of the following: 1. Se. F 2 2. As. I 3 3. Si. F 4 4. OBr 6 5. NH 4+ 6. NF 5

45 1. Draw a Lewis Dot diagram for the following 1. 2. 3. S-2 Ca Kr 2. Name the shape of the following, are they polar or nonpolar? 1. CH 4 2. H 2 O 3. NH 3

46 1. Longer bonds are ______ and have _______ bond energy than shorter bonds. 2. Covalent bonds are formed by the _______ of electrons. 3. A cation has a ______ charge. 4. The 3 Intermolecular Forces are… Put them in order of increasing strength. 5. The greater the IMF the ______ the melting point and boiling point. 6. What force gives water it’s unique properties? 7. What shape will be formed by the following: 1. NF 5 2. CCl 4 3. Ba. F 2

47 1. Name 3 properties of ionic and covalent compounds 2. How many p+, n 0, and e- in 40 K+? 3. What is KMT?

48 1. 2. Write the formula for the following ionic compounds: 1. Lithium phosphide 2. Calcium bromide 3. Boron nitride 4. Indium sulfide Name the following ionic compounds: 1. Al 2 S 3 2. K 2 O 3. Li 2 O 4. Ca. Br 2

49 • Give the name for the following compounds: • KBr • Mn 2 O 3 • S 2 O 3 • Cr. SO 3 • Give the formula for the following compounds: • lithium oxide • Vanadium IV oxide • Aluminum hydrogen carbonate • Iron III cyanide

50 1. Give the name for the following compounds: 1. Co. Br 3 2. Zn 3 P 2 3. Cu. SO 3 4. Li. NO 2 1. Give the formula for the following compounds: 1. Sodium hydroxide 2. Manganese IV carbonate 3. Potassium cyanide 4. Lead IV oxide

51 1. Give the name for the following compounds: 1. KBr 2. Mn 2 O 3 3. S 2 O 3 4. Cr. SO 3 1. Give the formula for the following compounds: 1. Dinitrogen monoxide 2. Dihydrogen monoxide 3. Vanadium V hydrogen carbonate 4. Iron III oxide

52 • Give the name for the following compounds: • Give the formula for the following compounds: • Be(NO 2)2 • Dinitrogen trioxide • H 3 PO 4 • Nitric acid • Si. O 2 • carbonic acid • PCl 3 • Dicarbon tetrabromide • H 2 SO 3 • Ammonium cyanide • Zn(Cl. O 3)2 • Hydroflouric acid

52 ANSWERS • Give the name for the following compounds: • Give the formula for the following compounds: • Be(NO 2)2 Berillium nitrite • Dinitrogen trioxide N 2 O 3 • H 3 PO 4 Phosphoric Acid • Nitric acid HNO 3 • Si. O 2 Silicon dioxide • carbonic acid H 2 CO 3 • PCl 3 Phosphorous trichloride • Dicarbon tetrabromide C 2 Br 4 • H 2 SO 3 Sulfurous Acid • Ammonium cyanide NH 4 CN • Zn(Cl. O 3)2 Zinc II chlorate • Hydroflouric acid HF

53 1. Give the name for the following compounds: 1. Give the formula for the following compounds: 1. Au. Br 1. Vanadium II Phosphite 2. H 2 SO 4 2. Sulfur hexachloride 3. N 2 O 3 3. Hydrosulfuric acid 4. Fe(OH)3 4. Aluminum hydroxide 5. CS 2 5. Tetraphosphorus trisulfide

53 ANSWERS • Give the name for the following compounds: • Give the formula for the following compounds: • Au. Br Gold I Bromide • Vanadium II Phosphite V 3(PO 3)2 • H 2 SO 4 Sulfuric Acid • Sulfur hexachloride SCl 6 • N 2 O 3 Dinitrogen trioxode • Hydrosulfuric acid H 2 S • Fe(OH)3 iron III hydroxide • Aluminum hydroxide Al(OH)3 • CS 2 carbon disulfide • Tetraphosphorus trisulfide P 4 S 3

54 Name the following compounds • NF 3 Give the formula for the following compounds • sulfurous acid • P 2 O 5 • dinitrogen trisulfide • HF • phosphorus pentabromide • S 4 N 2 • hydrobromic acid • H 3 PO 4 • dicarbon hexachloride

55 1. Wavelength and frequency are _____ related. 2. Light reflected from a green leaf has a frequency of 6. 12 x 1014 Hz what’s its wavelength? 3. 15. 34 g B=____ mol B 4. 2. 92 x 1023 atoms F =______g F 5. 2. 19 mol C=____g. C

56 FIND THE MOLAR MASS AND THEN CONVERT 1. 79. 85 g Fe 2 O 3 = ____ moles 2. 3. 01× 1024 formula units (NH 4)2 SO 4 = _____ g 3. 2. 00 mol of Sc. Cl 3 =______ g
57 FIND THE MOLAR MASS AND THEN CONVERT 1. 302. 7 g of Sc. Cl 3 =______ moles 2. 23 moles of H 2 O = ______ molecules

58 FIND THE MOLAR MASS AND THE % COMPOSITION 1. Na. OH 2. (NH 4)2 S

59 FIND THE MOLAR MASS AND THE % COMPOSITION Mn. Br 2 • 4 H 2 O

59 WHAT’S THE EMPIRICAL FORMULA OF A MOLECULE CONTAINING 65. 5% carbon, 5. 5% hydrogen, and 29. 0% oxygen?

60 FIND THE EMPIRICAL FORMULA OF THE FOLLOWING COMPOUND • 63. 5% Ag, 8. 2% N, 28. 3% O

61 Find the molecular formula: A compound with empirical formula C 2 HCl that has a molar mass of 302 g/mol

62 1. An unknown compound is found to contain 47. 0% K, 14. 5% C, and 38. 5% O. What is the empirical formula? If the experimental molar mass is 166. 22 g/mol, what is the molecular formula? 2. Find the molecular formula: A compound with empirical formula C 6 H 8 O that has a molar mass of 290 g/mol

63 1. A compound containing 5. 93% H and 94. 07% O has an experimental molar mass of 34. 01 g/mol. What is the empirical formula and the molecular formula of the compound? 2. Find the molar mass and the percent composition of Na. Br 3. How many moles are in 5. 55 g of CO 2?

64 1. The law of conservation of mass states that mass is not ____ nor ____ in chemical reactions. 2. ____ is the father of modern atomic theory. 3. ____ discovered the electron. 4. _____discovered the nucleus. 5. Name: Ca. C 03: ____________; Mn. Cl 2: _________; N 2 O 2: ________ 6. Write formulas for: aluminum chloride: _____; oxygen difluoride: _______; nickel III nitrate: ______

65 1. Hydrocarbons are organic compounds made of ____ & ____? 2. What is an allotrope. 3. What is the molecular formula of an alkane that has 17 C? 4. What is the name of C 7 H 16? 5. C generally makes _____ bonds.

66 1. C forms _____ covalent bonds. 2. _______ are hydrocarbons that only have single bonds. 3. _______ are hydrocarbons that have at least one double bond. 4. _______ are hydrocarbons that have at least one triple bond. 5. Draw 4 isomers of C 9 H 20.

67

68

69

70

71