Warm up Quote Nobody who ever gave their

Warm up Quote: “Nobody who ever gave their best regretted it. ” �George Stanley Halas, Sr. , nicknamed "Papa Bear" and "Mr. Everything", was a player, coach, owner and pioneer in professional American football. He was the iconic founder and owner of the National Football League's Chicago Bears. Riddle: I walked and at last I found it. I didn’t want it so I stopped and looked for it. When I found it I threw it away. �Possible letters: R N O T R E O O H I P R E I N

The Chemistry of Living Things
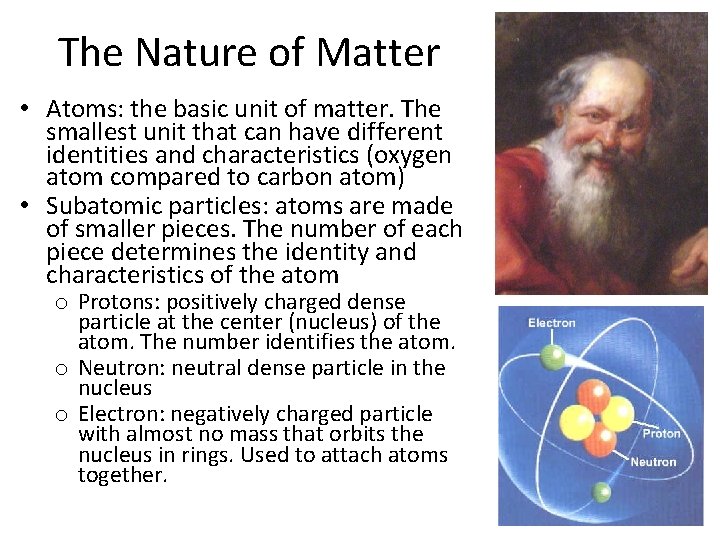
The Nature of Matter • Atoms: the basic unit of matter. The smallest unit that can have different identities and characteristics (oxygen atom compared to carbon atom) • Subatomic particles: atoms are made of smaller pieces. The number of each piece determines the identity and characteristics of the atom o Protons: positively charged dense particle at the center (nucleus) of the atom. The number identifies the atom. o Neutron: neutral dense particle in the nucleus o Electron: negatively charged particle with almost no mass that orbits the nucleus in rings. Used to attach atoms together.
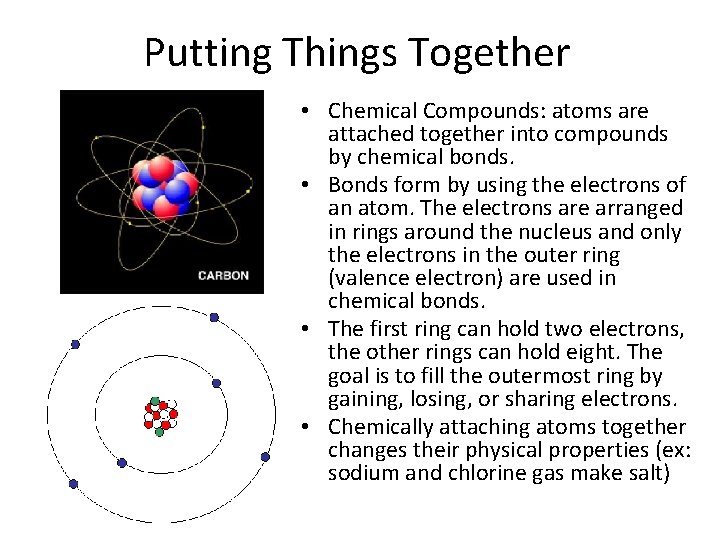
Putting Things Together • Chemical Compounds: atoms are attached together into compounds by chemical bonds. • Bonds form by using the electrons of an atom. The electrons are arranged in rings around the nucleus and only the electrons in the outer ring (valence electron) are used in chemical bonds. • The first ring can hold two electrons, the other rings can hold eight. The goal is to fill the outermost ring by gaining, losing, or sharing electrons. • Chemically attaching atoms together changes their physical properties (ex: sodium and chlorine gas make salt)
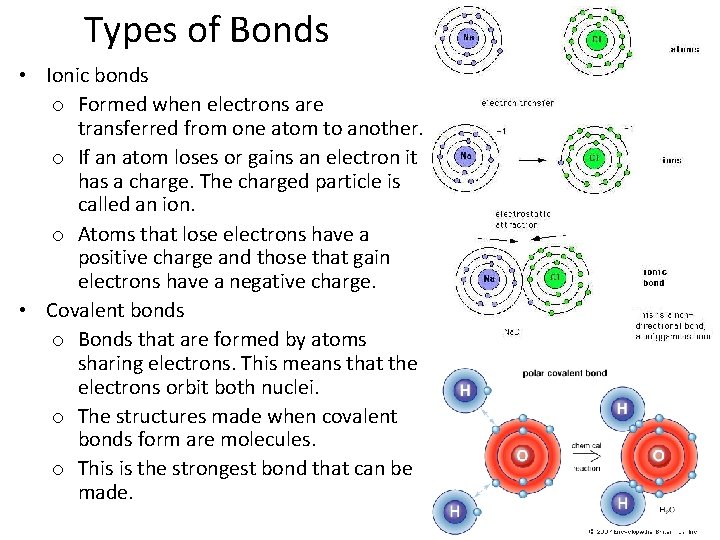
Types of Bonds • Ionic bonds o Formed when electrons are transferred from one atom to another. o If an atom loses or gains an electron it has a charge. The charged particle is called an ion. o Atoms that lose electrons have a positive charge and those that gain electrons have a negative charge. • Covalent bonds o Bonds that are formed by atoms sharing electrons. This means that the electrons orbit both nuclei. o The structures made when covalent bonds form are molecules. o This is the strongest bond that can be made.
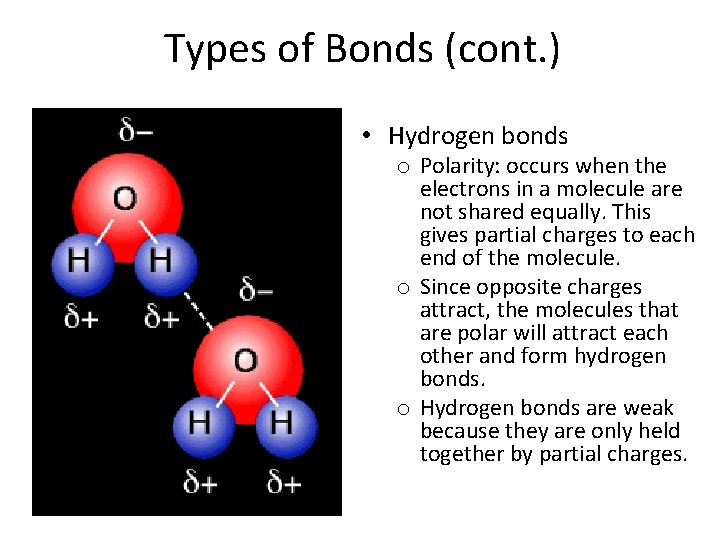
Types of Bonds (cont. ) • Hydrogen bonds o Polarity: occurs when the electrons in a molecule are not shared equally. This gives partial charges to each end of the molecule. o Since opposite charges attract, the molecules that are polar will attract each other and form hydrogen bonds. o Hydrogen bonds are weak because they are only held together by partial charges.

Properties of Water Caused by Hydrogen Bonds • Cohesion o An attraction between molecules of the same substance. o Water's cohesion causes molecules on the surface to be drawn inward. This is why water forms drops or beads on smooth surfaces. o Cohesion is also why water has so much surface tension. Surface tension allows insects to walk on the surface of bodies of water
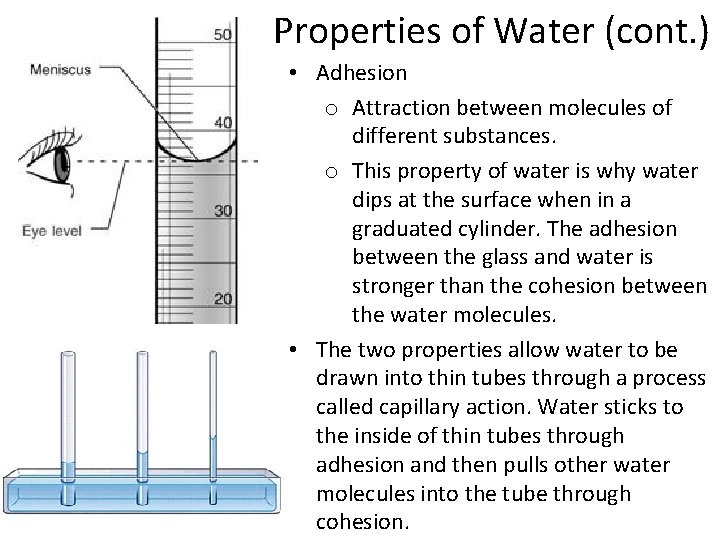
Properties of Water (cont. ) • Adhesion o Attraction between molecules of different substances. o This property of water is why water dips at the surface when in a graduated cylinder. The adhesion between the glass and water is stronger than the cohesion between the water molecules. • The two properties allow water to be drawn into thin tubes through a process called capillary action. Water sticks to the inside of thin tubes through adhesion and then pulls other water molecules into the tube through cohesion.

Mixtures • Mixtures occur when two or more molecules are physically mixed but not chemically attached. There are two kinds: o Solution: one substance is dissolved in another. The particles are evenly distributed throughout. Solutes are the substances dissolved while solvents are substances in which solutes are dissolved. Waters polarity allows it to dissolve ionic compounds and other polar compounds. o Suspension: when the movement of the water keeps the particles suspended. Ex) Blood cells are suspended in a plasma

Warm Up • Quote: – “Nothing will work unless you do. ” • Maya Angelou was an American author, poet, dancer, actress, and singer. She published seven autobiographies, three books of essays, and several books of poetry, and was credited with a list of plays, movies, and television shows spanning over 50 years. • Riddle: – The person that builds it sells it. The person that buys it doesn’t use it. The person that does use it never sees it. What is it?
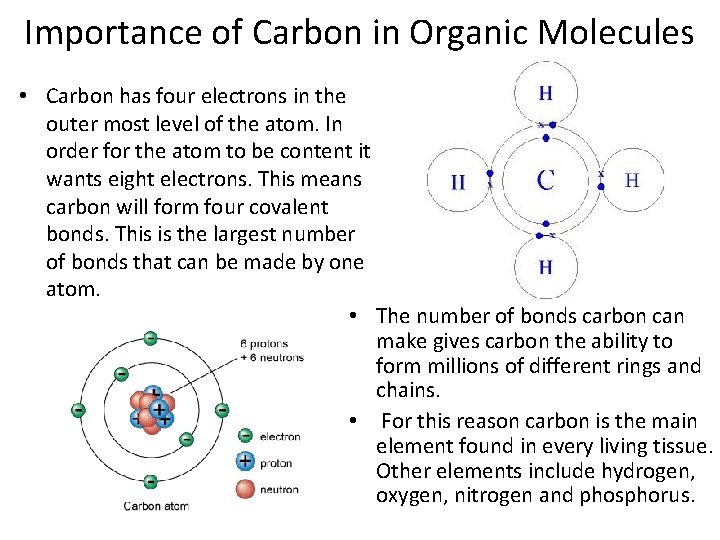
Importance of Carbon in Organic Molecules • Carbon has four electrons in the outer most level of the atom. In order for the atom to be content it wants eight electrons. This means carbon will form four covalent bonds. This is the largest number of bonds that can be made by one atom. • The number of bonds carbon can make gives carbon the ability to form millions of different rings and chains. • For this reason carbon is the main element found in every living tissue. Other elements include hydrogen, oxygen, nitrogen and phosphorus.
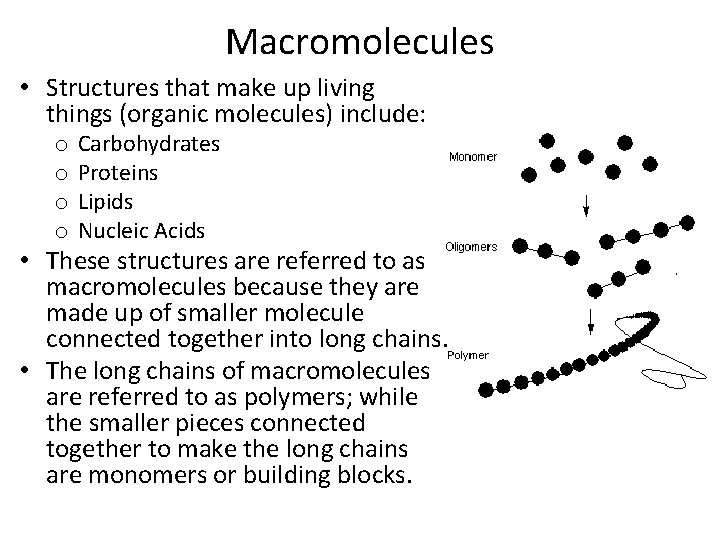
Macromolecules • Structures that make up living things (organic molecules) include: o o Carbohydrates Proteins Lipids Nucleic Acids • These structures are referred to as macromolecules because they are made up of smaller molecule connected together into long chains. • The long chains of macromolecules are referred to as polymers; while the smaller pieces connected together to make the long chains are monomers or building blocks.
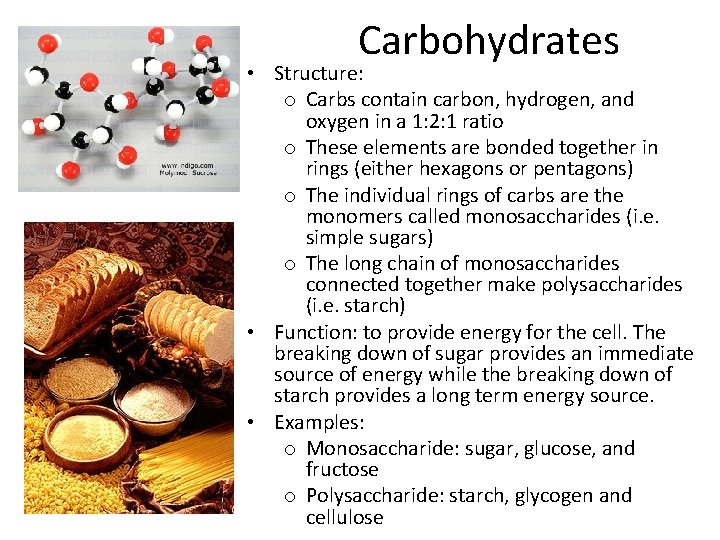
Carbohydrates • Structure: o Carbs contain carbon, hydrogen, and oxygen in a 1: 2: 1 ratio o These elements are bonded together in rings (either hexagons or pentagons) o The individual rings of carbs are the monomers called monosaccharides (i. e. simple sugars) o The long chain of monosaccharides connected together make polysaccharides (i. e. starch) • Function: to provide energy for the cell. The breaking down of sugar provides an immediate source of energy while the breaking down of starch provides a long term energy source. • Examples: o Monosaccharide: sugar, glucose, and fructose o Polysaccharide: starch, glycogen and cellulose
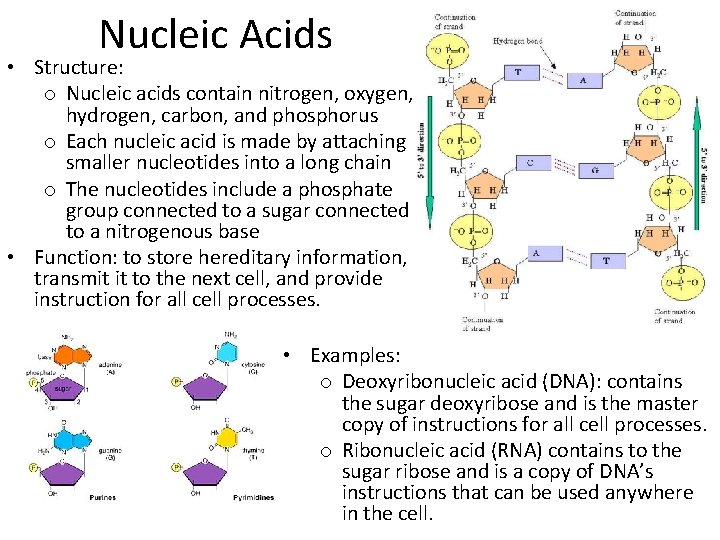
Nucleic Acids • Structure: o Nucleic acids contain nitrogen, oxygen, hydrogen, carbon, and phosphorus o Each nucleic acid is made by attaching smaller nucleotides into a long chain o The nucleotides include a phosphate group connected to a sugar connected to a nitrogenous base • Function: to store hereditary information, transmit it to the next cell, and provide instruction for all cell processes. • Examples: o Deoxyribonucleic acid (DNA): contains the sugar deoxyribose and is the master copy of instructions for all cell processes. o Ribonucleic acid (RNA) contains to the sugar ribose and is a copy of DNA’s instructions that can be used anywhere in the cell.
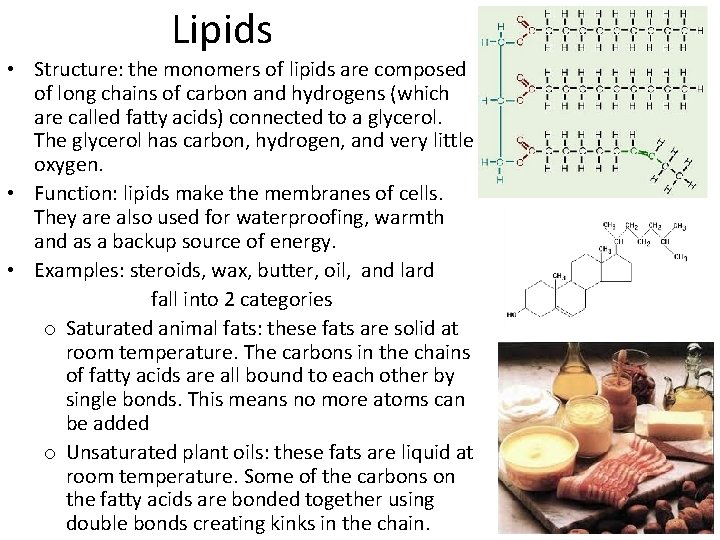
Lipids • Structure: the monomers of lipids are composed of long chains of carbon and hydrogens (which are called fatty acids) connected to a glycerol. The glycerol has carbon, hydrogen, and very little oxygen. • Function: lipids make the membranes of cells. They are also used for waterproofing, warmth and as a backup source of energy. • Examples: steroids, wax, butter, oil, and lard fall into 2 categories o Saturated animal fats: these fats are solid at room temperature. The carbons in the chains of fatty acids are all bound to each other by single bonds. This means no more atoms can be added o Unsaturated plant oils: these fats are liquid at room temperature. Some of the carbons on the fatty acids are bonded together using double bonds creating kinks in the chain.
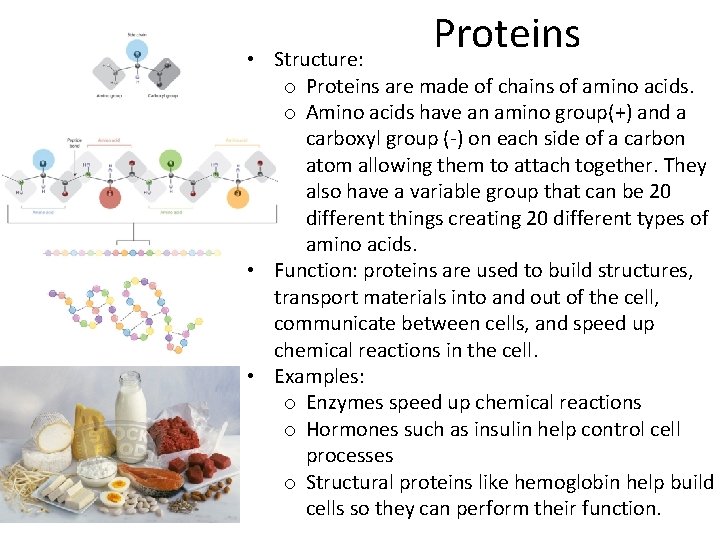
Proteins • Structure: o Proteins are made of chains of amino acids. o Amino acids have an amino group(+) and a carboxyl group (-) on each side of a carbon atom allowing them to attach together. They also have a variable group that can be 20 different things creating 20 different types of amino acids. • Function: proteins are used to build structures, transport materials into and out of the cell, communicate between cells, and speed up chemical reactions in the cell. • Examples: o Enzymes speed up chemical reactions o Hormones such as insulin help control cell processes o Structural proteins like hemoglobin help build cells so they can perform their function.
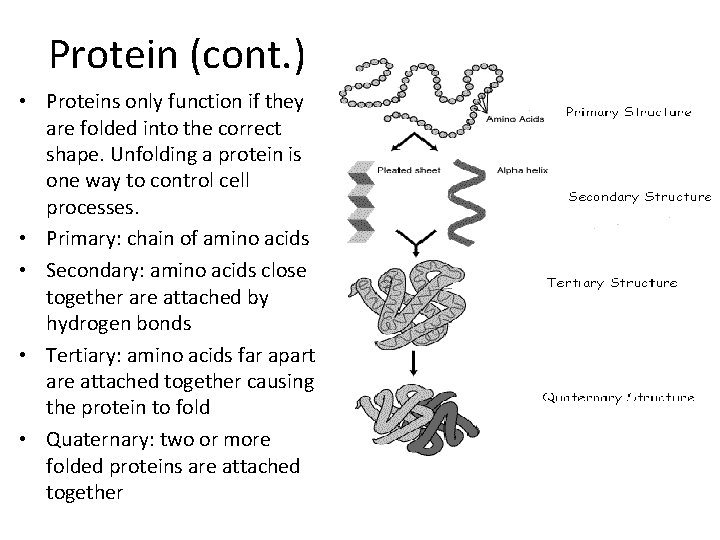
Protein (cont. ) • Proteins only function if they are folded into the correct shape. Unfolding a protein is one way to control cell processes. • Primary: chain of amino acids • Secondary: amino acids close together are attached by hydrogen bonds • Tertiary: amino acids far apart are attached together causing the protein to fold • Quaternary: two or more folded proteins are attached together

• Quote Warm Up – “Nothing is softer or more flexible than water, yet nothing can resist it. ” • Lao Tzu was a philosopher and poet of ancient China. He is known as the reputed author of the Tao Te Ching and the founder of philosophical Taoism, and as a deity in religious Taoism and traditional Chinese religions. • Riddle – Double my number, I’m less than a score. Half of my number is less than four. Add one to my double when bakers are near. Days of the week are still greater, I fear.
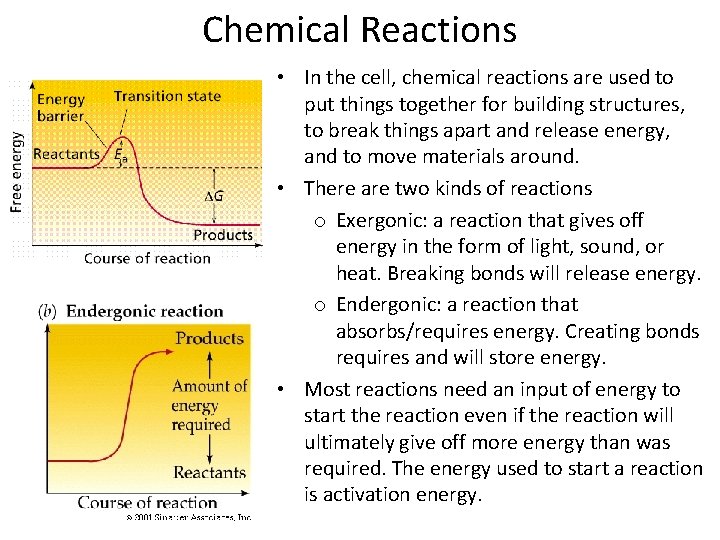
Chemical Reactions • In the cell, chemical reactions are used to put things together for building structures, to break things apart and release energy, and to move materials around. • There are two kinds of reactions o Exergonic: a reaction that gives off energy in the form of light, sound, or heat. Breaking bonds will release energy. o Endergonic: a reaction that absorbs/requires energy. Creating bonds requires and will store energy. • Most reactions need an input of energy to start the reaction even if the reaction will ultimately give off more energy than was required. The energy used to start a reaction is activation energy.
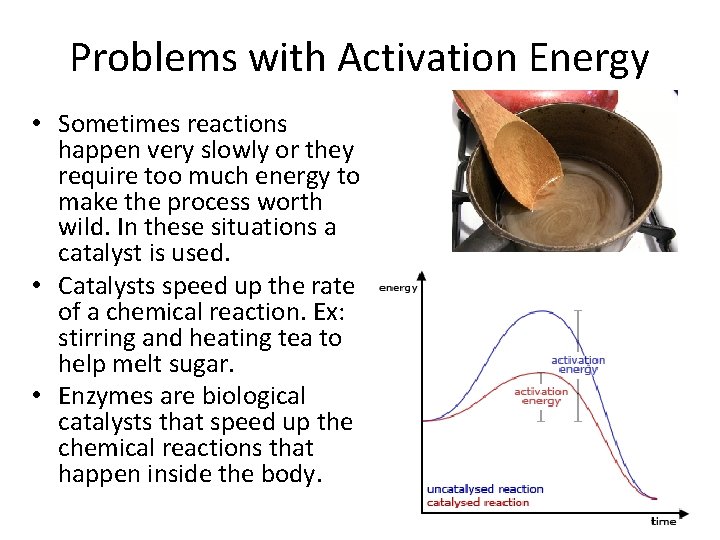
Problems with Activation Energy • Sometimes reactions happen very slowly or they require too much energy to make the process worth wild. In these situations a catalyst is used. • Catalysts speed up the rate of a chemical reaction. Ex: stirring and heating tea to help melt sugar. • Enzymes are biological catalysts that speed up the chemical reactions that happen inside the body.
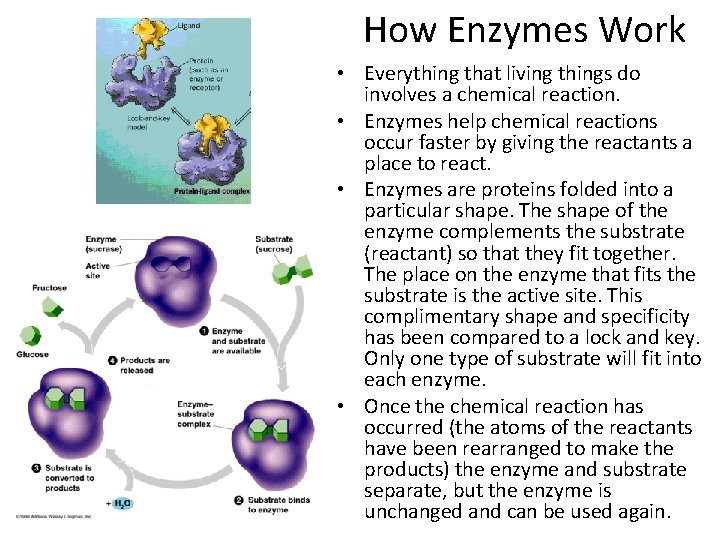
How Enzymes Work • Everything that living things do involves a chemical reaction. • Enzymes help chemical reactions occur faster by giving the reactants a place to react. • Enzymes are proteins folded into a particular shape. The shape of the enzyme complements the substrate (reactant) so that they fit together. The place on the enzyme that fits the substrate is the active site. This complimentary shape and specificity has been compared to a lock and key. Only one type of substrate will fit into each enzyme. • Once the chemical reaction has occurred (the atoms of the reactants have been rearranged to make the products) the enzyme and substrate separate, but the enzyme is unchanged and can be used again.
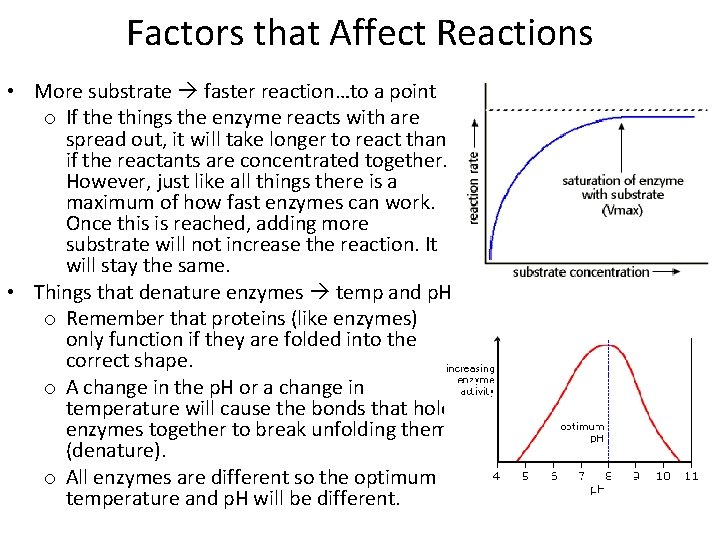
Factors that Affect Reactions • More substrate faster reaction…to a point o If the things the enzyme reacts with are spread out, it will take longer to react than if the reactants are concentrated together. However, just like all things there is a maximum of how fast enzymes can work. Once this is reached, adding more substrate will not increase the reaction. It will stay the same. • Things that denature enzymes temp and p. H o Remember that proteins (like enzymes) only function if they are folded into the correct shape. o A change in the p. H or a change in temperature will cause the bonds that hold enzymes together to break unfolding them (denature). o All enzymes are different so the optimum temperature and p. H will be different.
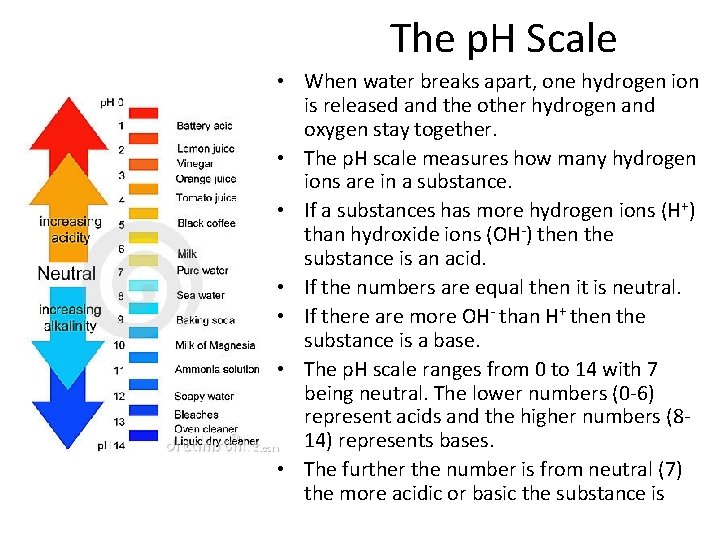
The p. H Scale • When water breaks apart, one hydrogen ion is released and the other hydrogen and oxygen stay together. • The p. H scale measures how many hydrogen ions are in a substance. • If a substances has more hydrogen ions (H+) than hydroxide ions (OH-) then the substance is an acid. • If the numbers are equal then it is neutral. • If there are more OH- than H+ then the substance is a base. • The p. H scale ranges from 0 to 14 with 7 being neutral. The lower numbers (0 -6) represent acids and the higher numbers (814) represents bases. • The further the number is from neutral (7) the more acidic or basic the substance is

p. H and the Body • Since enzymes work best at a specific p. H the bodies of living things have to control the p. H level. Eating something that is acidic or basic cannot be allowed to change the p. H of the body. • In order to control the p. H levels the cells make weak acids and bases that will react if a strong acid or base is added to prevent it from changing the p. H. These substances are called buffers.

p. H Lab Questions ON THE BACK 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) Define acid. What is the p. H range of an acid? What is a strong acid’s p. H? What is a weak acid’s p. H? Define acidic. Define base. What is the p. H range of a base? What is a strong base’s p. H? What is a weak base’s p. H? Define alkaline.
- Slides: 26