Warm Up n Write the longhand electron configuration

















- Slides: 27

Warm Up n Write the longhand electron configuration for the following elements: n n n Cr F Sr
Periodic Trends

Part I – Atomic Size

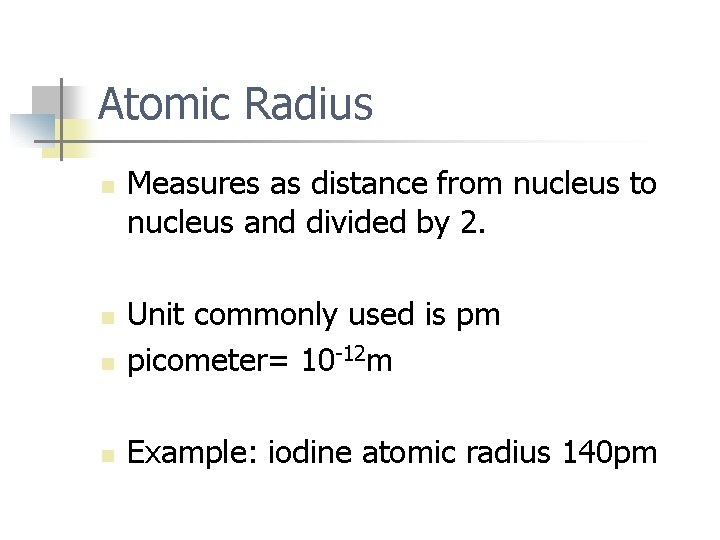
Atomic Radius n Measures as distance from nucleus to nucleus and divided by 2. n Unit commonly used is pm picometer= 10 -12 m n Example: iodine atomic radius 140 pm n

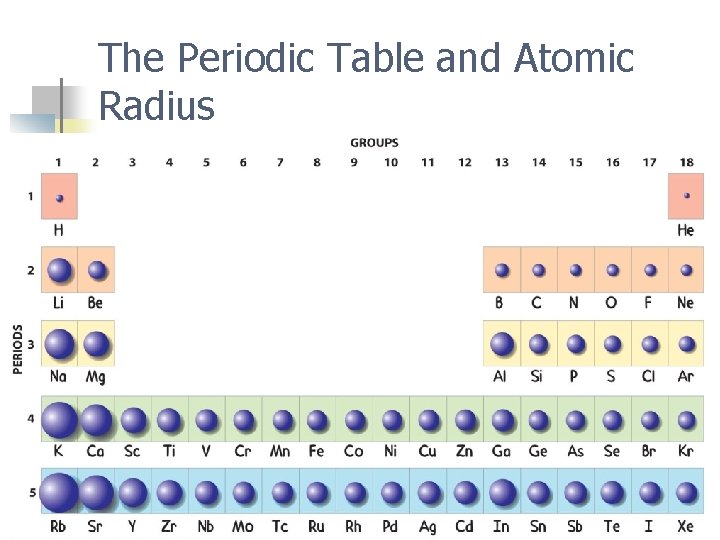
How does atomic radius change across a period? n It is smaller to the right. n Why? n More protons in the nucleus higher electrical force pulls electrons closer to nucleus.

How does atomic radius change down a group? n It is larger down the group. n Why? n Valence electrons are at higher energy levels and are not bound as tightly to the nucleus because they are screened or shielded ( pushed away) by other electrons in inner levels.

The Periodic Table and Atomic Radius

Example: Which is larger: a lithium atom or a fluorine atom? A lithium atom

Example: n n Which is larger: an arsenic atom or a sulfur atom? An arsenic atom

Part II – Ionization Energy

Ionization energy n Ionization energy is the amount of energy needed to remove an electron from a gaseous atom. n n First ionization energy – Second ionization energy – 1+ 2+

Ion n Positive ion ---removal of electron n Negative ion--- addition of electron

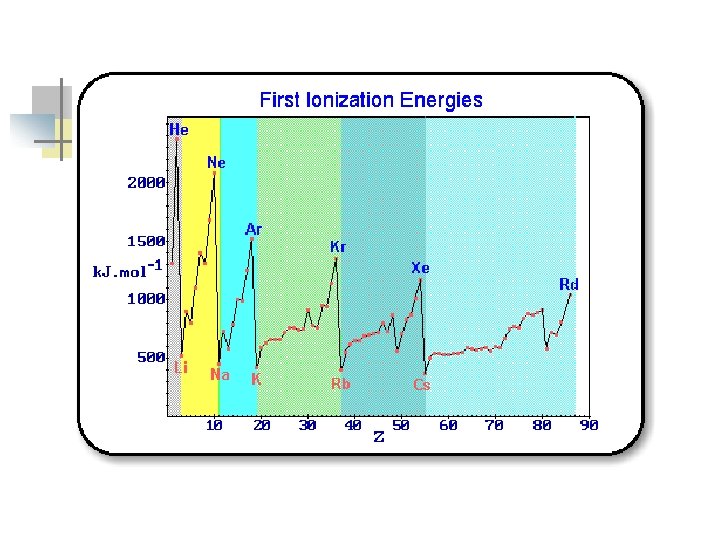
How does ionization energy change down a group? n n The first ionization energy decreases as you move down a group. Why? n n The size of the atom increases. Electron is further from the nucleus.

How does ionization energy change across a period? n n The first ionization energy increases as you move from left to right across a period. Why? n n Nuclear charge increases while shielding is constant. Attraction of the electron to the nucleus increases.


Ionic size n n Metallic elements easily lose electrons. Non-metals more readily gain electrons. How does losing or gaining an electron effect the size of the atom (ion) ?

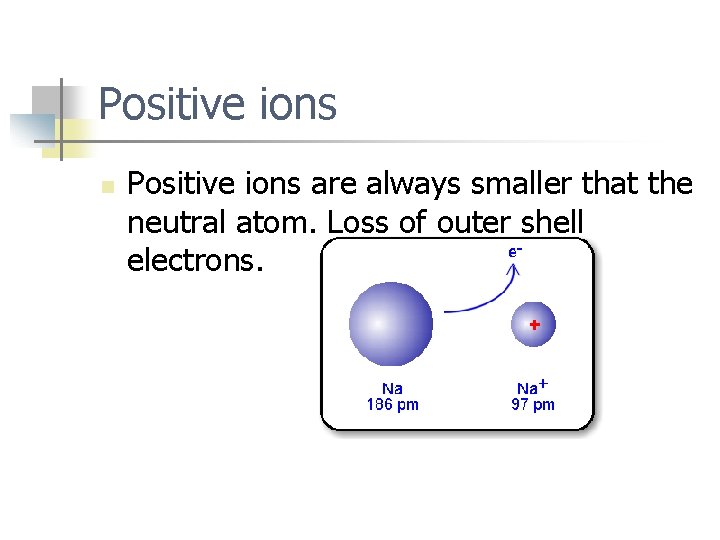
Positive ions n Positive ions are always smaller that the neutral atom. Loss of outer shell electrons.

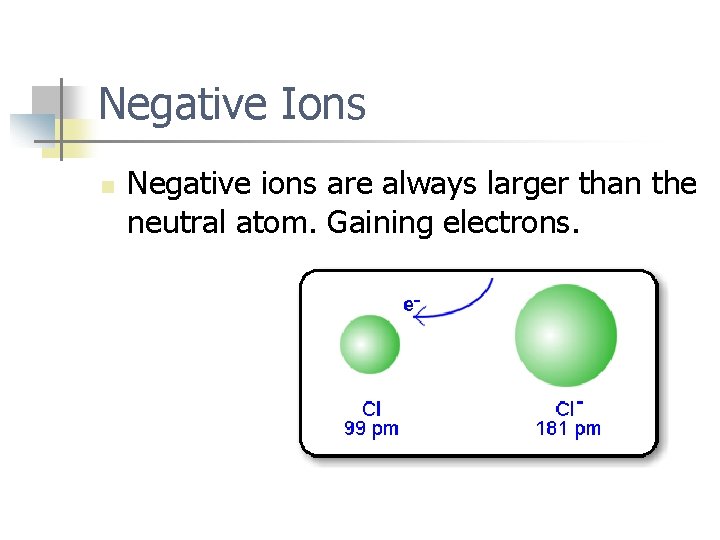
Negative Ions n Negative ions are always larger than the neutral atom. Gaining electrons.

Ion size trends in periods. n n Going from left to right there is a decrease in size of positive ions. Starting with group 5, there is sharp increase followed by a decrease in the size of the anion as you move from left to right.

Ion size trends in columns. n Ion size increases as you move down a column for both positive and negative ions

Warm Up (hand in for a quiz grade) n In your own terms: n n State and Explain the trend for atomic radius going across a period and down a group. Do the same for Ionization energy.

Electronegativity: the ability of an atom in a bond to pull on the electron. (Linus Pauling)

Electronegativity n n n When electrons are shared by two atoms a covalent bond is formed. When the atoms are the same they pull on the electrons equally. Example, H-H. When the atoms are different, the atoms pull on the electrons unevenly. Example, HCl

Trends in Electronegativity n n Electronegativity generally decreases as you move down a group. Electronegativity of the representative elements (Group A elements) increases as you move across a period.

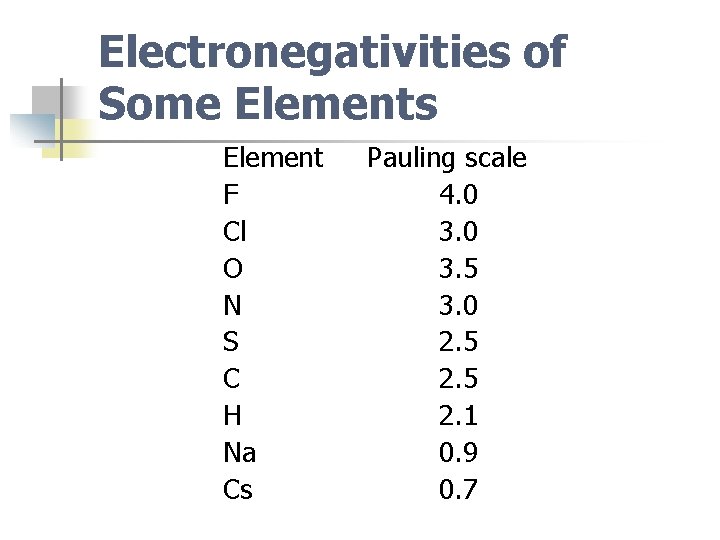
Electronegativities of Some Elements Element F Cl O N S C H Na Cs Pauling scale 4. 0 3. 5 3. 0 2. 5 2. 1 0. 9 0. 7

Note n n Most electronegative element is F (EN 4. 0) Least electronegative stable element is Cs (EN 0. 7)

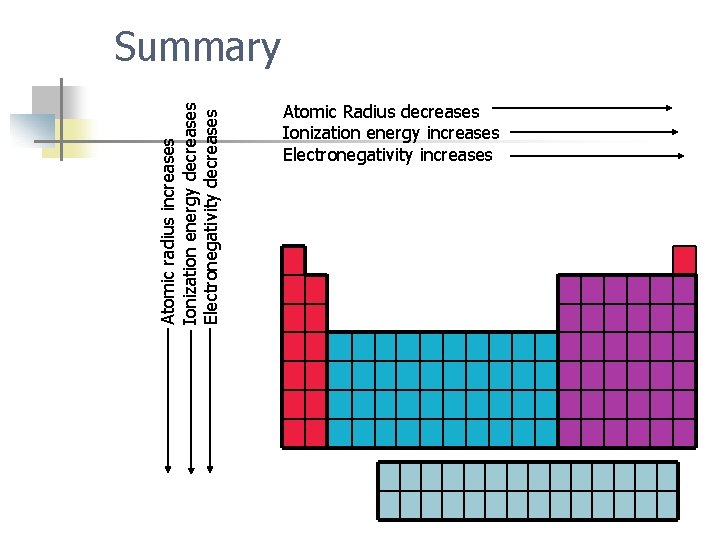
Atomic radius increases Ionization energy decreases Electronegativity decreases Summary Atomic Radius decreases Ionization energy increases Electronegativity increases