Warm Up n Describe how the periodic table

+ Warm Up n. Describe how the periodic table is arranged.

+ Warm Up As an introduction to the periodic table, describe how YOU believe the periodic table is arranged. **Continue to work on your periodic table activity from Wednesday**
+ The Periodic Table: Organizing the Elements Chapter 6

+Warm Up! n What is the law that states the properties of elements are periodic functions of their atomic numbers? What are the elements -to the left of the stair case -on the staircase -to the right of the stair case What is the difference between metals, nonmetals, and metalloids? **Turn in your flame lab and candium lab to the bin!!**

+ Dobereiner’s Triad (1829) n Organized the elements into groups of three (triads) according to similar properties. n Example: Halogen Triad – Chlorine, Bromine, & Iodine n n React easily with metals. n Atomic mass of bromine is approximately halfway between the atomic masses of chlorine and iodine. PROBLEM: Not all known elements could be grouped into triads. (Iron, Manganese, Nickel, Cobalt, etc. )

+ Mendeleev’s Periodic Table (1869) n Dmitri Mendeleev, Russian chemist n Organized ~60 elements by INCREASING ATOMIC MASS. n Elements arranged in groups according to repeating properties. n Predicted properties of undiscovered elements. n Mendeleev is credited with the creation of the Periodic Table.
Mendeleev’s Periodicity of Elements +
Mendeleev’s First Periodic Table of Elements +
+ Mendeleev’s Periodic Table of Elements
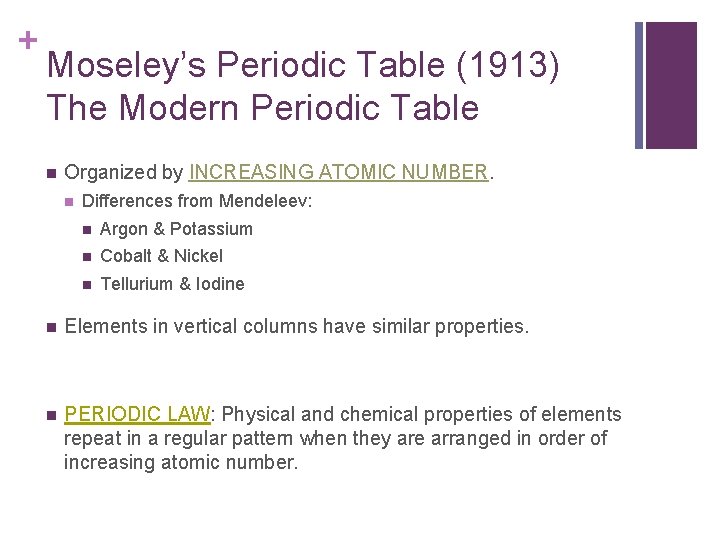
+ Moseley’s Periodic Table (1913) The Modern Periodic Table n Organized by INCREASING ATOMIC NUMBER. n Differences from Mendeleev: n Argon & Potassium n Cobalt & Nickel n Tellurium & Iodine n Elements in vertical columns have similar properties. n PERIODIC LAW: Physical and chemical properties of elements repeat in a regular pattern when they are arranged in order of increasing atomic number.

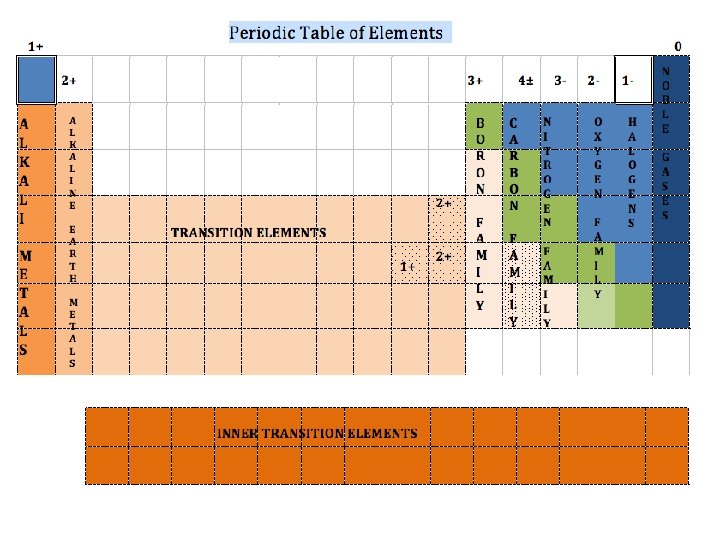
+ Understanding the Periodic Table n Vertical Columns: Groups or Families n Horizontal n Divided n n n Rows: Periods into three main categories: Metals: Left Side Nonmetals: Right Side Metalloids (Semi-Metals): Along Zig-Zag Line

+ Metals n Left of zig-zag line n Properties: n Excellent conductors of heat and electricity n High luster (shiny) n All are solids at room temperature (except Mercury) n Many are malleable & ductile. n n Malleable: can be hammered into a sheet n Ductile: can be drawn into a wire Valence electrons: n Most have 1, 2, or 3 n Held loosely (LOSE electrons to form compounds).

+ Metals n Transition & Inner Transition elements are metals n Inner Transition Elements located below the table n Lanthanides – Rare Earth Elements n Actinides – All are Radioactive & those beyond Uranium are not found in nature.

+ Nonmetals n Right of zig-zag line n Properties: n Many are gases at room temperature. Those that are solid at room temp. lack luster. n Carbon & Phosphorus - solids n Bromine & Iodine – liquids n Poor conductors of heat and electric current. n Brittle n Valence electrons: n Most have 5, 6, 7, or 8 n Held tightly (GAIN or SHARE electrons to form compounds).

+ Metalloids (Semi-metals) n Along the zig-zag line (except Aluminum) n Properties of both metals & nonmetals. n Some are Semiconductors: silicon, germanium, arsenic n n Silicon is generally a poor conductor of electricity. If a small amount of Boron is mixed in the silicon (doping), the mixture is a good conductor. (Used to make computer chips).
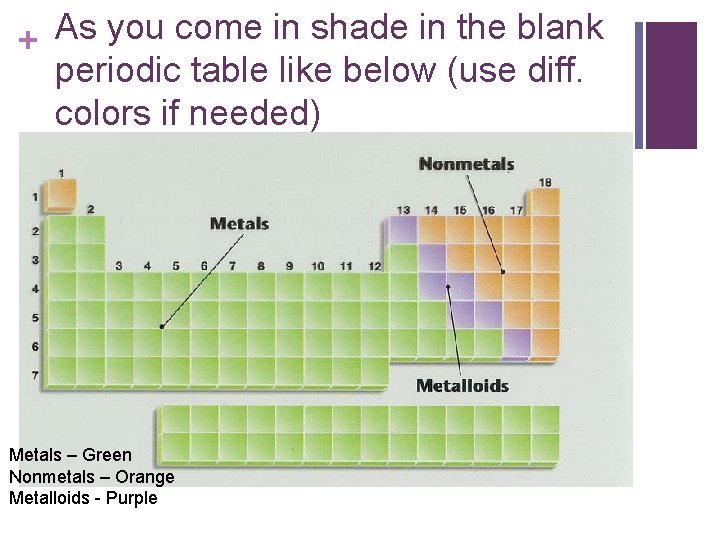
As you come in shade in the blank + periodic table like below (use diff. colors if needed) Metals – Green Nonmetals – Orange Metalloids - Purple
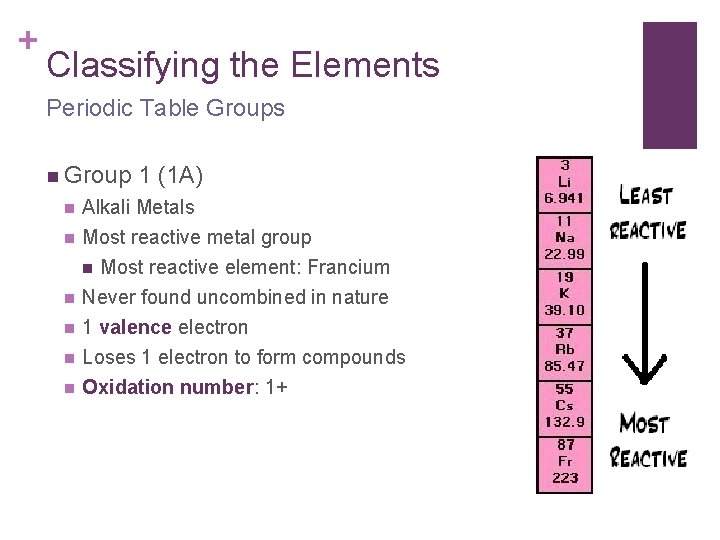
+ Classifying the Elements Periodic Table Groups n Group 1 (1 A) n Alkali Metals n Most reactive metal group n Most reactive element: Francium Never found uncombined in nature 1 valence electron Loses 1 electron to form compounds Oxidation number: 1+ n n
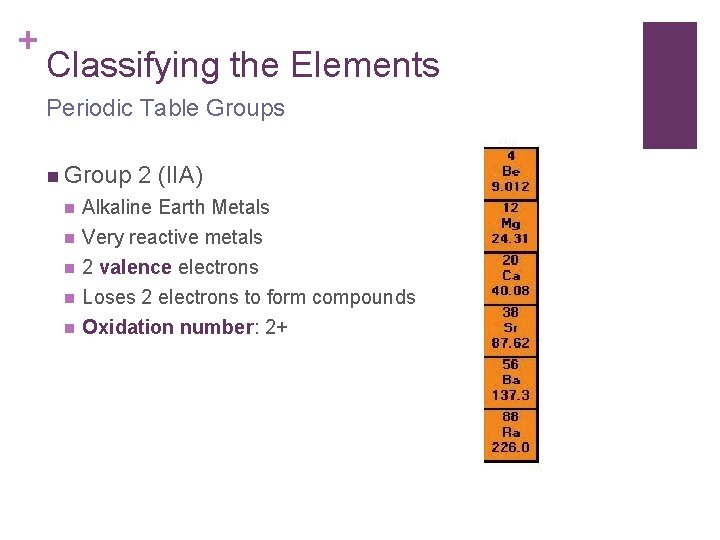
+ Classifying the Elements Periodic Table Groups n Group 2 (IIA) n Alkaline Earth Metals n Very reactive metals 2 valence electrons Loses 2 electrons to form compounds Oxidation number: 2+ n n n
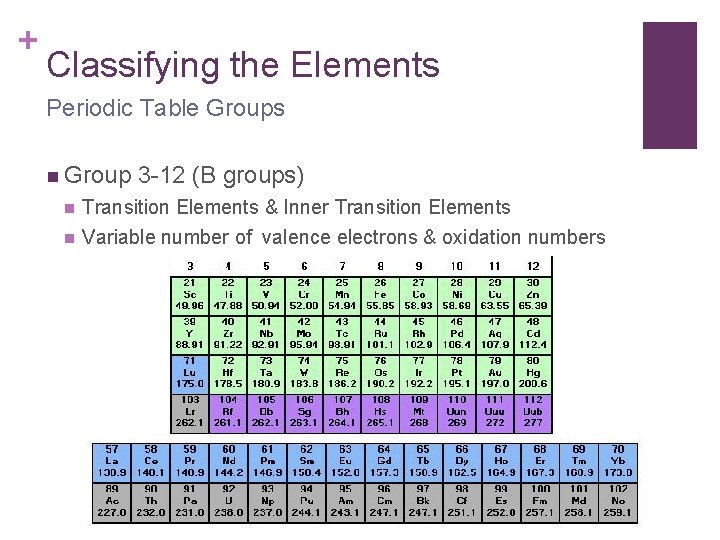
+ Classifying the Elements Periodic Table Groups n Group 3 -12 (B groups) n Transition Elements & Inner Transition Elements n Variable number of valence electrons & oxidation numbers
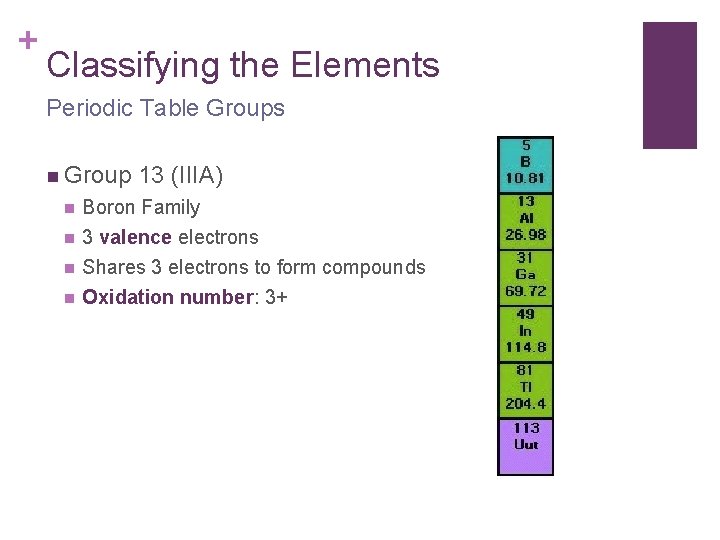
+ Classifying the Elements Periodic Table Groups n Group 13 (IIIA) n Boron Family n 3 valence electrons Shares 3 electrons to form compounds Oxidation number: 3+ n n
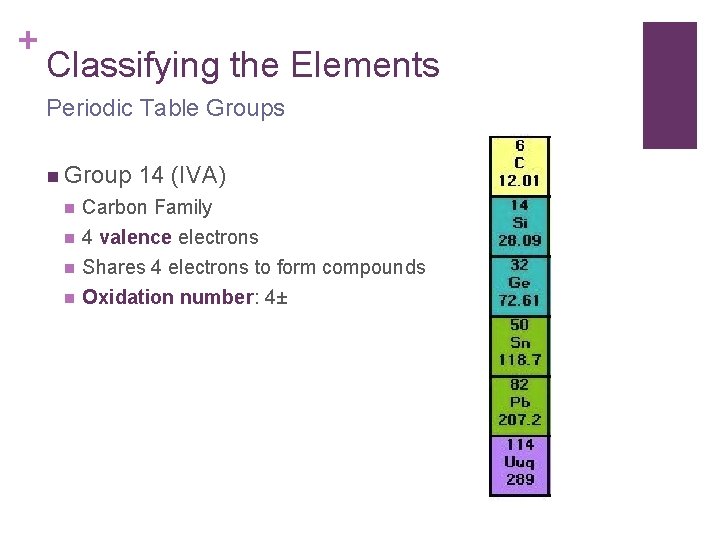
+ Classifying the Elements Periodic Table Groups n Group 14 (IVA) n Carbon Family n 4 valence electrons Shares 4 electrons to form compounds Oxidation number: 4± n n
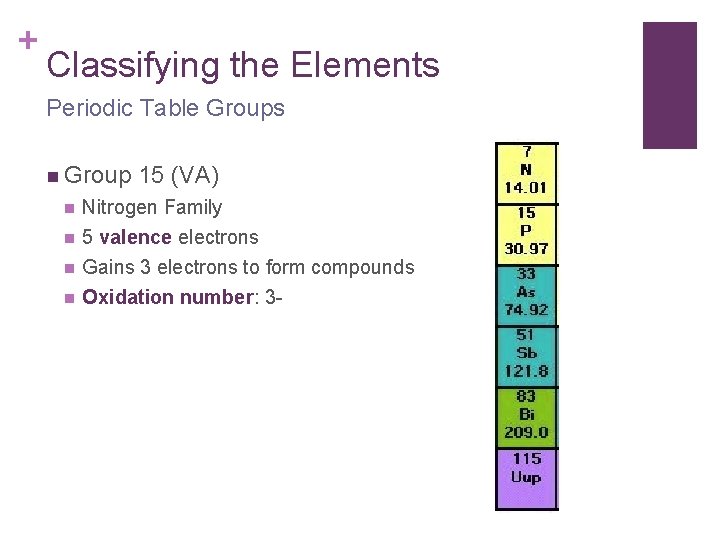
+ Classifying the Elements Periodic Table Groups n Group 15 (VA) n Nitrogen Family n 5 valence electrons Gains 3 electrons to form compounds Oxidation number: 3 - n n
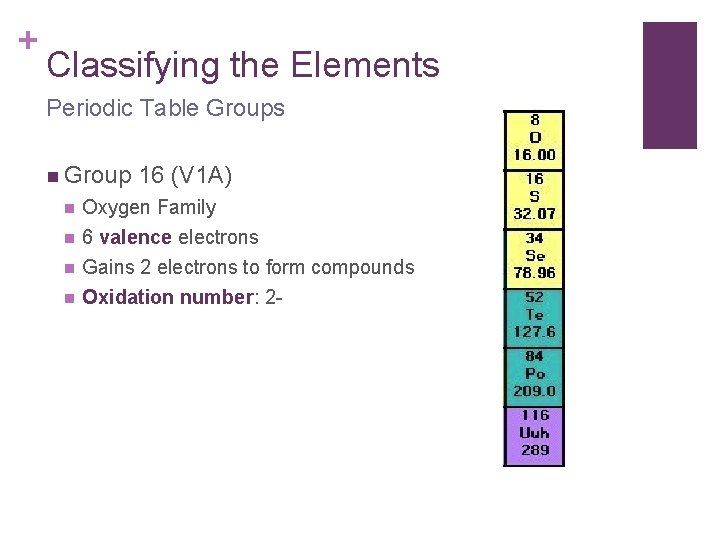
+ Classifying the Elements Periodic Table Groups n Group 16 (V 1 A) n Oxygen Family n 6 valence electrons Gains 2 electrons to form compounds Oxidation number: 2 - n n
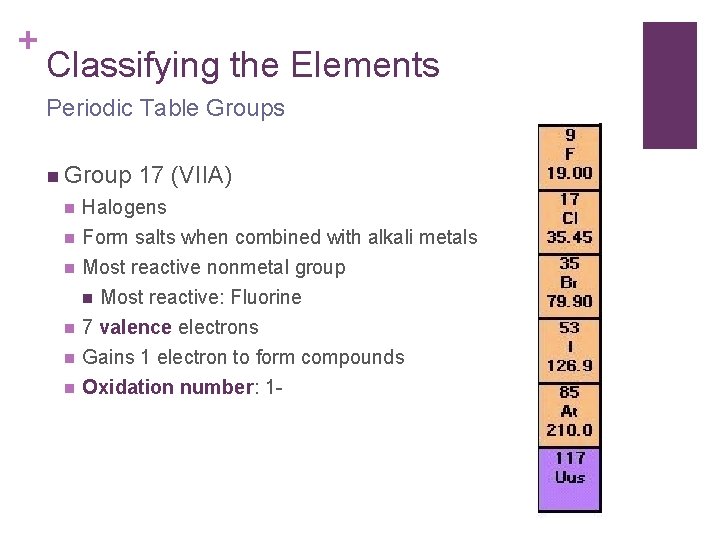
+ Classifying the Elements Periodic Table Groups n Group 17 (VIIA) n Halogens n Form salts when combined with alkali metals Most reactive nonmetal group n Most reactive: Fluorine 7 valence electrons Gains 1 electron to form compounds Oxidation number: 1 - n n
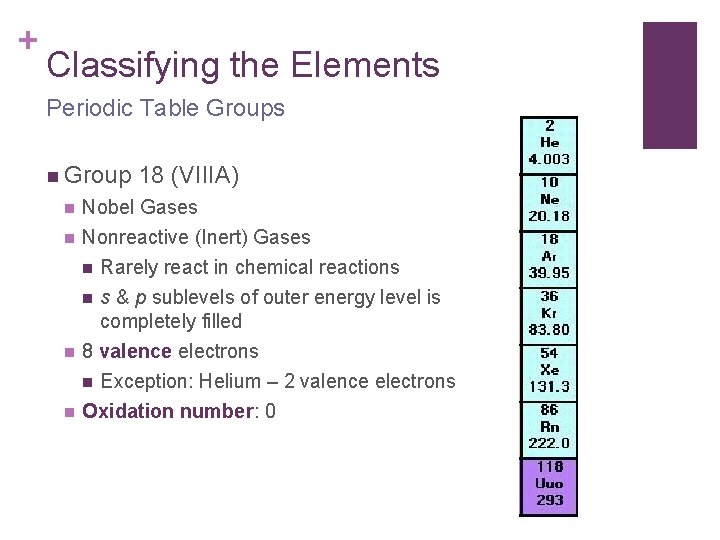
+ Classifying the Elements Periodic Table Groups n Group 18 (VIIIA) n Nobel Gases n Nonreactive (Inert) Gases n Rarely react in chemical reactions n s & p sublevels of outer energy level is completely filled 8 valence electrons n Exception: Helium – 2 valence electrons n n Oxidation number: 0
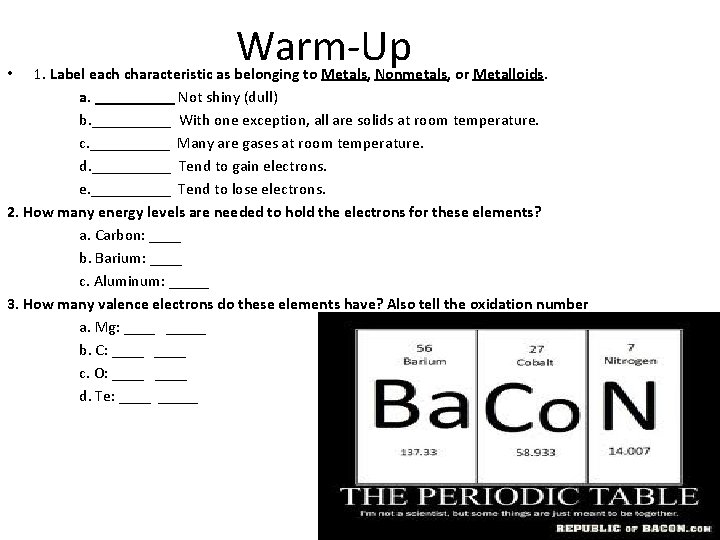
Warm-Up 1. Label each characteristic as belonging to Metals, Nonmetals, or Metalloids. a. _____ Not shiny (dull) b. _____ With one exception, all are solids at room temperature. c. _____ Many are gases at room temperature. d. _____ Tend to gain electrons. e. _____ Tend to lose electrons. 2. How many energy levels are needed to hold the electrons for these elements? a. Carbon: ____ b. Barium: ____ c. Aluminum: _____ 3. How many valence electrons do these elements have? Also tell the oxidation number a. Mg: _____ b. C: ____ c. O: ____ d. Te: _____ •

Periodic Table Trends Atomic Size • Ionization Energy • Electronegativity •

Trends in Atomic Size Atomic Radius ◦ Half the diameter of an atom ◦ Measured in picometers (pm) Trend: ◦ Atomic size INCREASES down a group WHY? Energy levels are added down a group ◦ Atomic size DECREASES across a period WHY? Protons are added moving across a period, increasing the pull of attraction from the nucleus and pulling electrons closer.
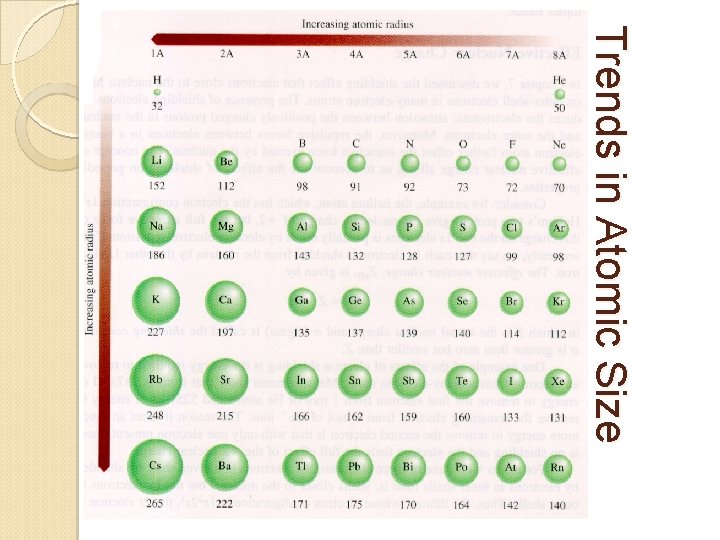
Trends in Atomic Size

Practice: Which atom is larger? Explain why. ANSWER: • Barium or Magnesium? • Barium • Farther down the group • Sodium or Sulfur? • Sodium • Farther left in the period • Oxygen or Chlorine? • Chlorine • Farther down the group • Shielding effect • more energy levels
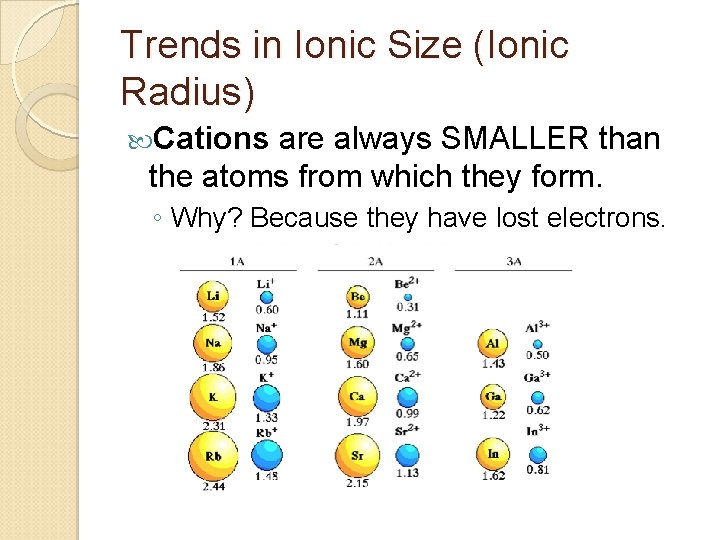
Trends in Ionic Size (Ionic Radius) Cations are always SMALLER than the atoms from which they form. ◦ Why? Because they have lost electrons.
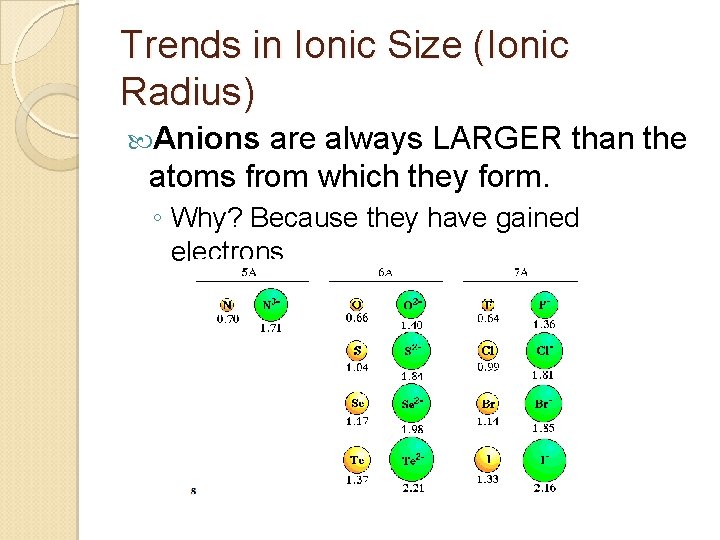
Trends in Ionic Size (Ionic Radius) Anions are always LARGER than the atoms from which they form. ◦ Why? Because they have gained electrons.

Trends in Ionic Size (Ionic Radius) Trend: ◦ Ionic size INCREASES down a group WHY? Energy levels are added down a group ◦ Ionic size DECREASES across a period WHY? Protons are added moving across a period, increasing the pull of attraction from the nucleus and pulling electrons closer. SAME TREND AS ATOMIC RADIUS!
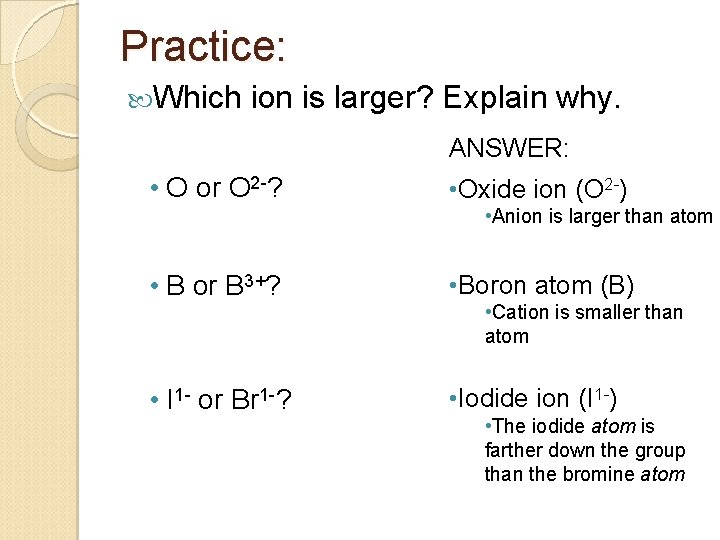
Practice: Which ion is larger? Explain why. ANSWER: • O or O 2 -? • Oxide ion (O 2 -) • Anion is larger than atom • B or B 3+? • Boron atom (B) • I 1 - or Br 1 -? • Iodide ion (I 1 -) • Cation is smaller than atom • The iodide atom is farther down the group than the bromine atom

Trends in Ionization Energy ◦ Energy needed to REMOVE an electron from an atom. ◦ First Ionization Energy required to remove the first electron ◦ Second Ionization Energy required to remove the second electron ◦ Third Ionization Energy required to remove third electron

Trends in Ionization Energy Trend: ◦ Ionization Energy DECREASES down a group WHY? Electrons that occupy higher energy levels are easier to remove because they are farther from the nucleus. ◦ Ionization Energy INCREASES across a period WHY? As protons are added across a period, the electrons are harder to remove because the nucleus is pulling them with a greater force.
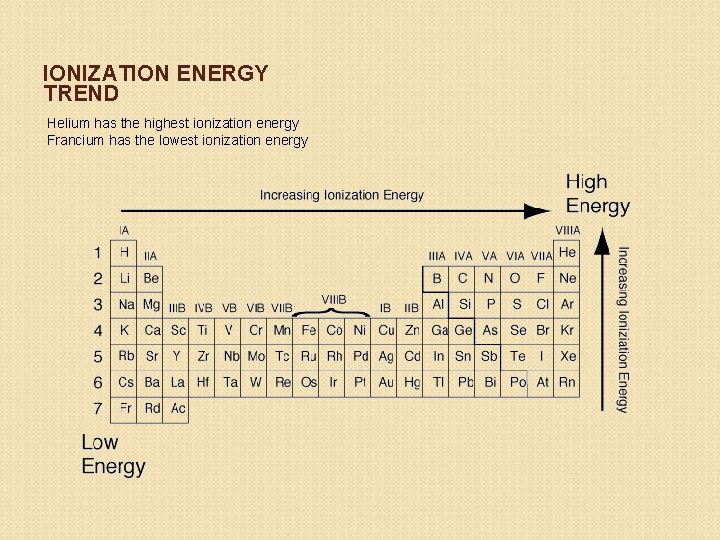
IONIZATION ENERGY TREND Helium has the highest ionization energy Francium has the lowest ionization energy

Practice: Which has a greater first ionization energy? ANSWER: • Potassium or Sulfur? • Sulfur • Farther to the right • Lithium or Francium? • Lithium • Farther to the top • Carbon or Phosphorus? • Farther to the top • Fewer energy levels, stronger force of attraction

Trends in Electronegativity ◦ Ability of an atom to attract electrons when the atom is in a compound. When looking at this trend, we IGNORE the noble gases because they to not have the ability to attract electrons when in a compound since they have a filled s & p sublevels.
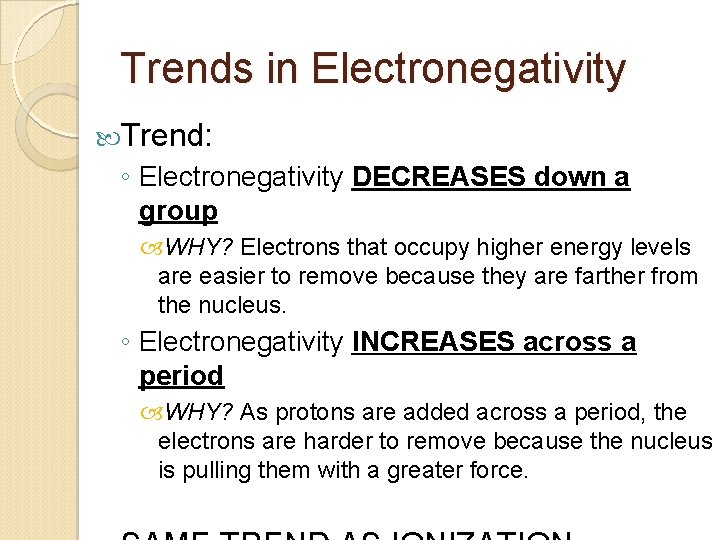
Trends in Electronegativity Trend: ◦ Electronegativity DECREASES down a group WHY? Electrons that occupy higher energy levels are easier to remove because they are farther from the nucleus. ◦ Electronegativity INCREASES across a period WHY? As protons are added across a period, the electrons are harder to remove because the nucleus is pulling them with a greater force.
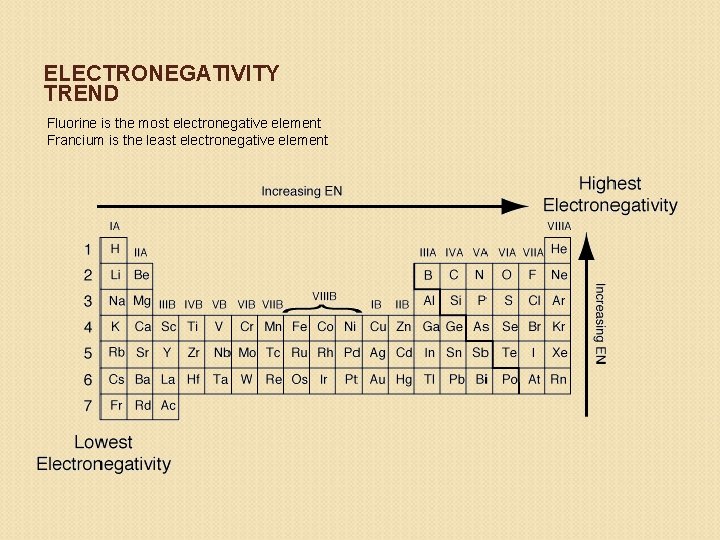
ELECTRONEGATIVITY TREND Fluorine is the most electronegative element Francium is the least electronegative element
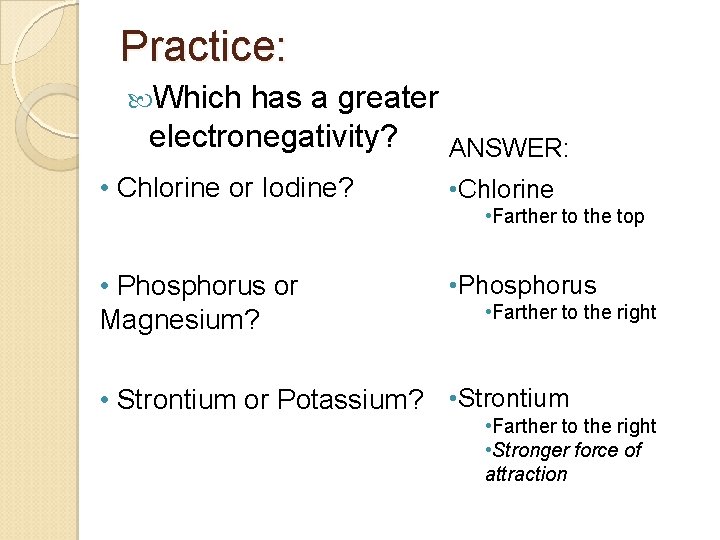
Practice: Which has a greater electronegativity? ANSWER: • Chlorine or Iodine? • Chlorine • Farther to the top • Phosphorus or Magnesium? • Phosphorus • Farther to the right • Strontium or Potassium? • Strontium • Farther to the right • Stronger force of attraction
Electrons
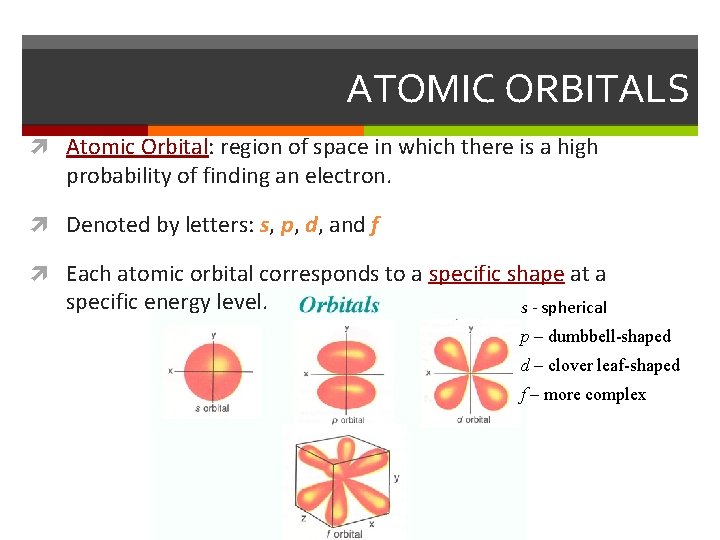
ATOMIC ORBITALS Atomic Orbital: region of space in which there is a high probability of finding an electron. Denoted by letters: s, p, d, and f Each atomic orbital corresponds to a specific shape at a specific energy level. s - spherical p – dumbbell-shaped d – clover leaf-shaped f – more complex
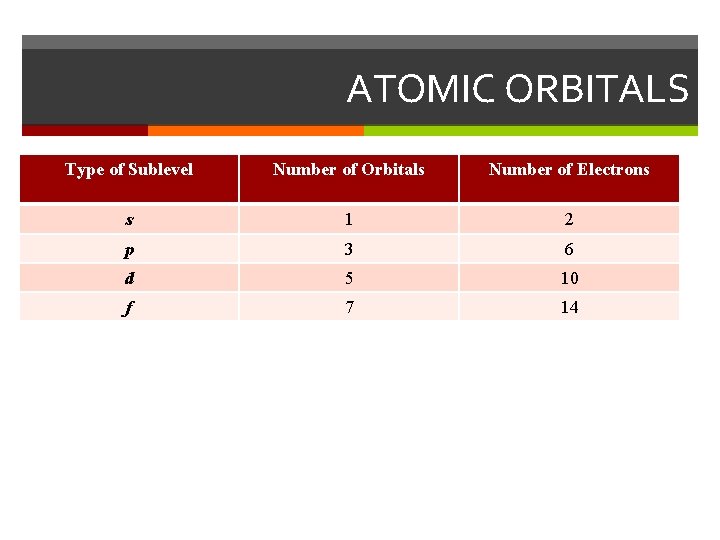
ATOMIC ORBITALS Type of Sublevel Number of Orbitals Number of Electrons s 1 2 p 3 6 d 5 10 f 7 14
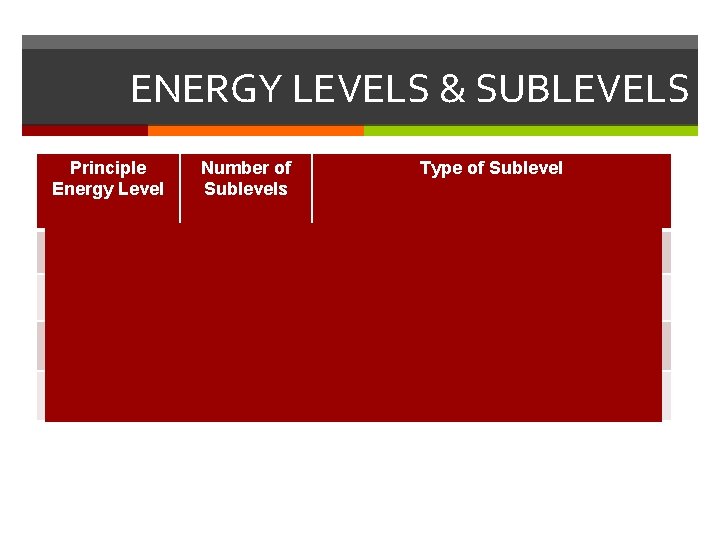
ENERGY LEVELS & SUBLEVELS Principle Energy Level Number of Sublevels Type of Sublevel n=1 1 1 s (1 orbital) n=2 2 2 s (1 orbital), 2 p (3 orbitals) n=3 3 3 s (1 orbital), 3 p (3 orbitals), 3 d (5 orbitals) n=4 4 4 s (1 orbital), 4 p (3 orbitals), 4 d (5 orbitals), 4 f (7 orbitals)
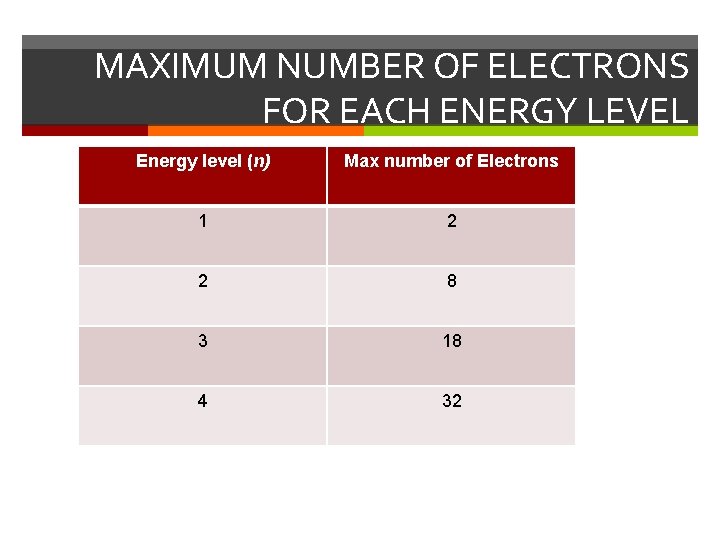
MAXIMUM NUMBER OF ELECTRONS FOR EACH ENERGY LEVEL Energy level (n) Max number of Electrons 1 2 2 8 3 18 4 32
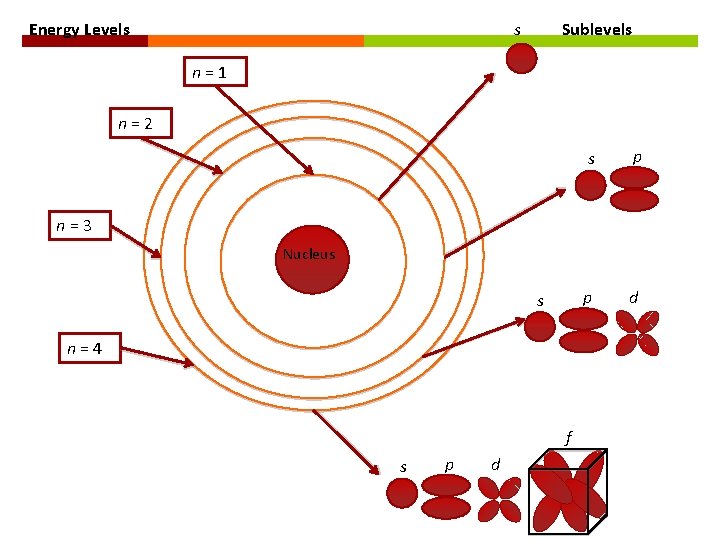
Energy Levels s Sublevels n=1 n=2 s p p d n=3 Nucleus s n=4 f s p d
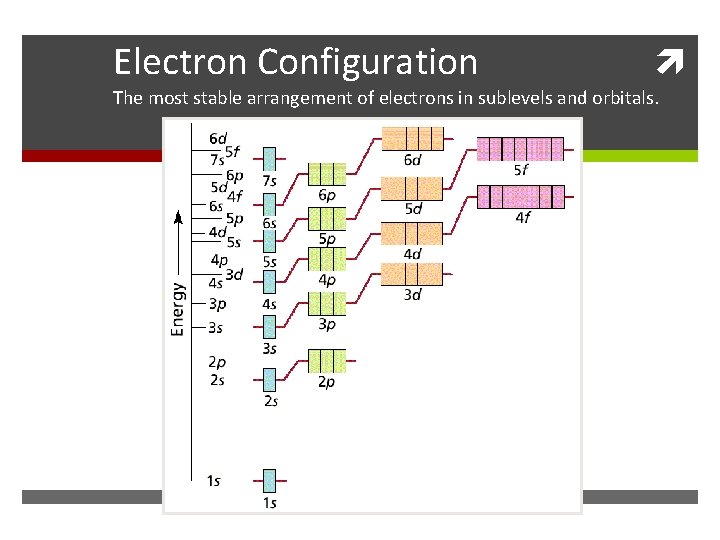
Electron Configuration The most stable arrangement of electrons in sublevels and orbitals.
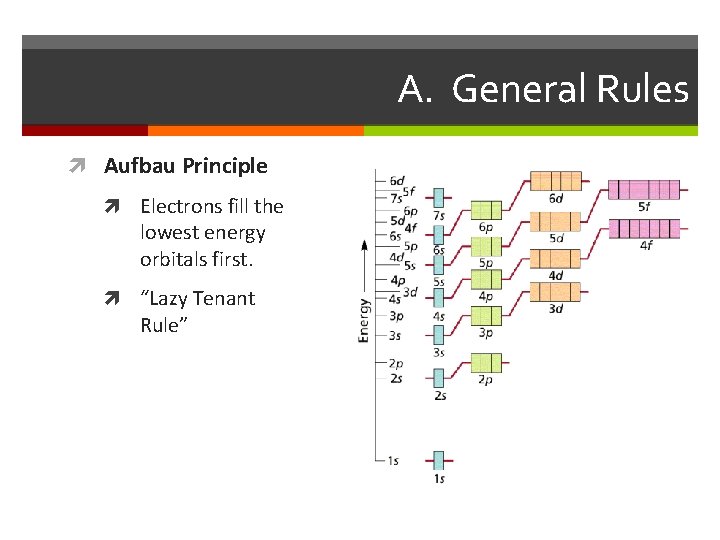
A. General Rules Aufbau Principle Electrons fill the lowest energy orbitals first. “Lazy Tenant Rule”

A. General Rules Pauli Exclusion Principle Each orbital can hold TWO electrons with opposite spins.
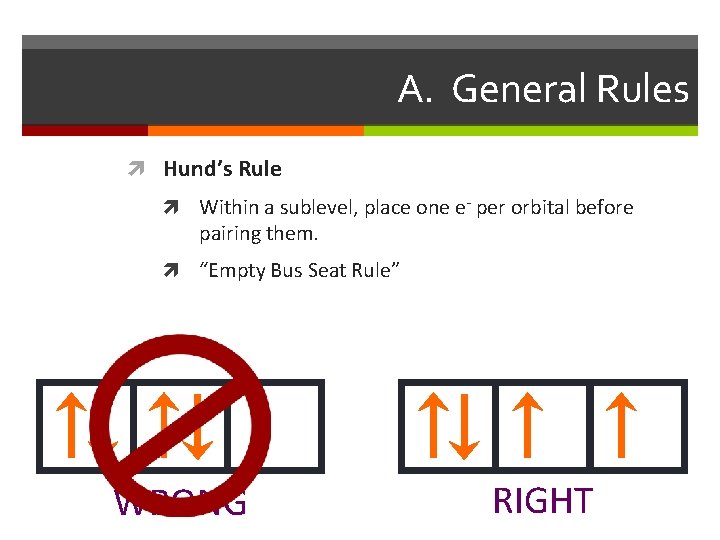
A. General Rules Hund’s Rule Within a sublevel, place one e- per orbital before pairing them. “Empty Bus Seat Rule” WRONG RIGHT
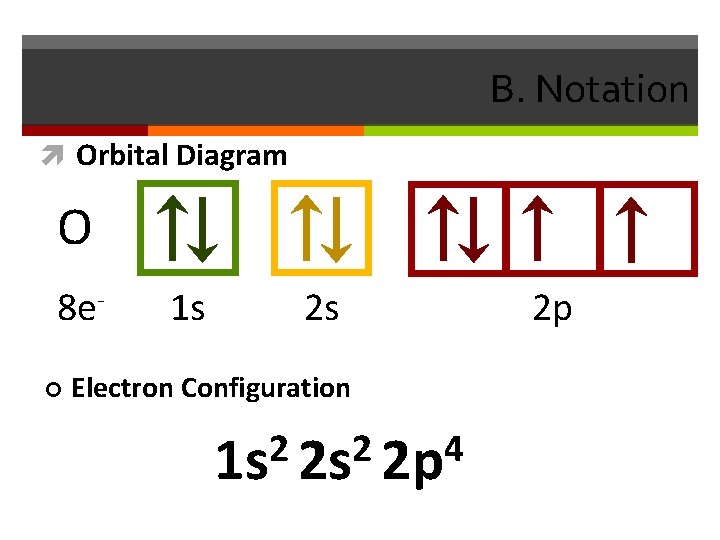
B. Notation Orbital Diagram O 8 e¢ 1 s 2 s Electron Configuration 2 2 4 1 s 2 s 2 p 2 p
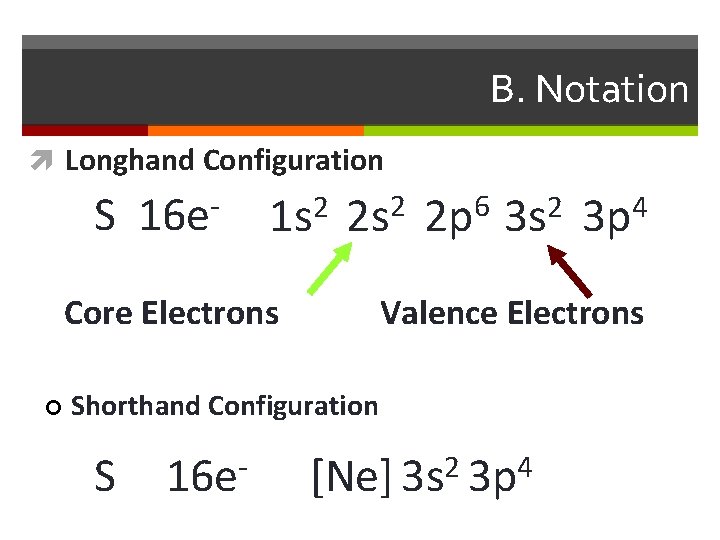
B. Notation Longhand Configuration S 16 e 1 s 2 2 p 6 3 s 2 3 p 4 Core Electrons ¢ Valence Electrons Shorthand Configuration S 16 e- [Ne] 3 s 2 3 p 4
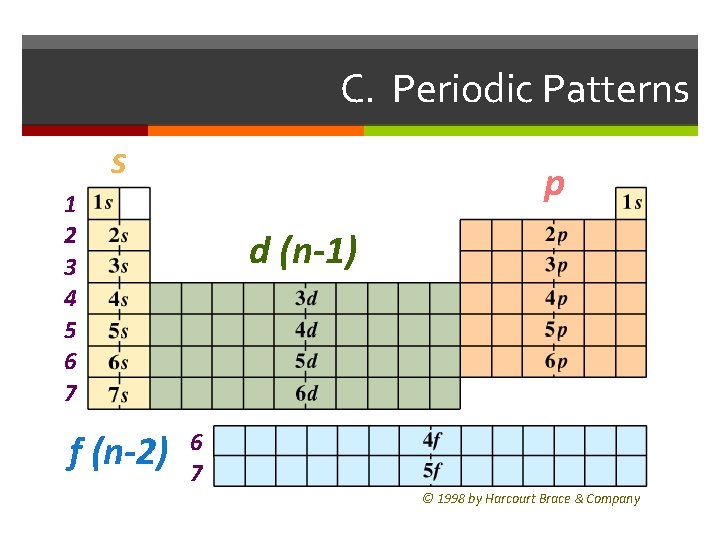
C. Periodic Patterns s p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company
C. Periodic Patterns Period # energy level (subtract for d & f) A/B Group # total # of valence e Valence electrons are electrons in the outermost energy level. Determines the chemical and physical properties of an element. Column within sublevel block # of e- in sublevel
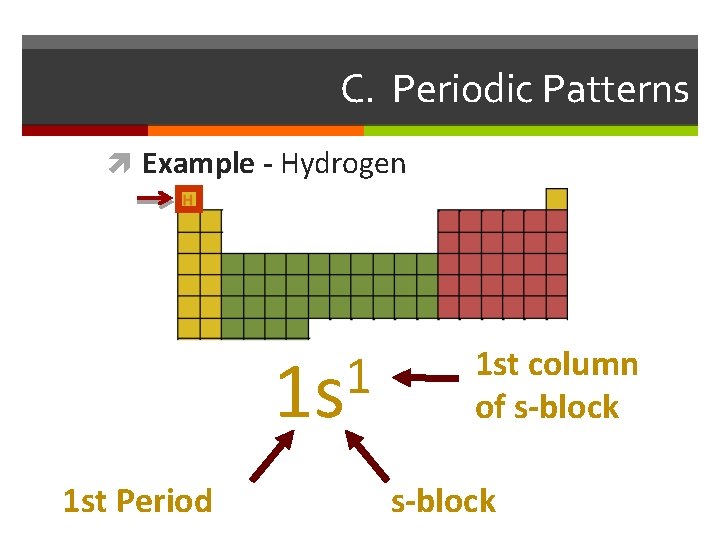
C. Periodic Patterns Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block

C. Periodic Patterns Shorthand Configuration Core e-: Go up one row and over to the Noble Gas. Valence e-: On the next row, fill in the # of e- in each sublevel.
![C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2 C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2](http://slidetodoc.com/presentation_image/1217ebe797978732600d66eaf1282435/image-60.jpg)
C. Periodic Patterns Example - Germanium [Ar] 2 4 s 10 3 d 2 4 p
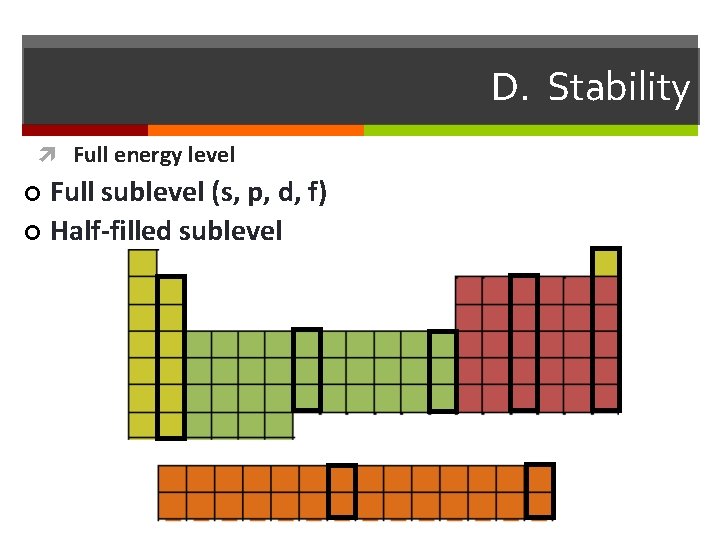
D. Stability Full energy level Full sublevel (s, p, d, f) ¢ Half-filled sublevel ¢
![D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3 D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3](http://slidetodoc.com/presentation_image/1217ebe797978732600d66eaf1282435/image-62.jpg)
D. Stability Electron Configuration Exceptions l l Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 Copper gains stability with a full d-sublevel.
![D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2 D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2](http://slidetodoc.com/presentation_image/1217ebe797978732600d66eaf1282435/image-63.jpg)
D. Stability Electron Configuration Exceptions l Chromium EXPECT: ACTUALLY: l [Ar] 4 s 2 3 d 4 [Ar] 4 s 1 3 d 5 Chromium gains stability with a half-filled dsublevel.
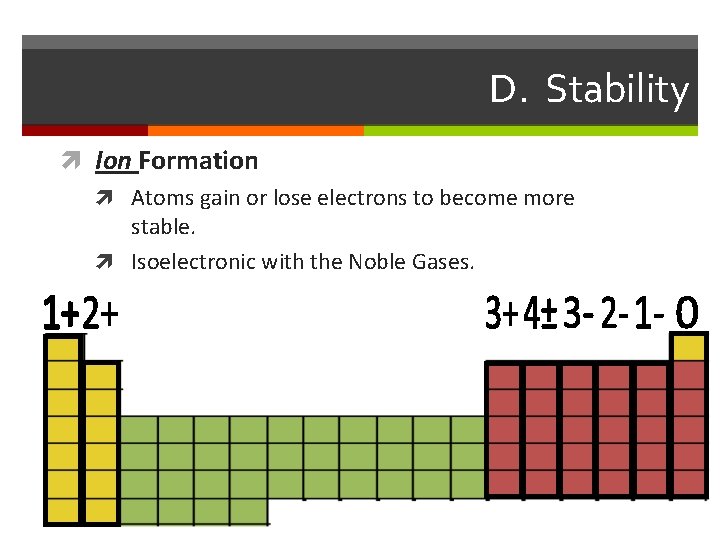
D. Stability Ion Formation Atoms gain or lose electrons to become more stable. Isoelectronic with the Noble Gases.
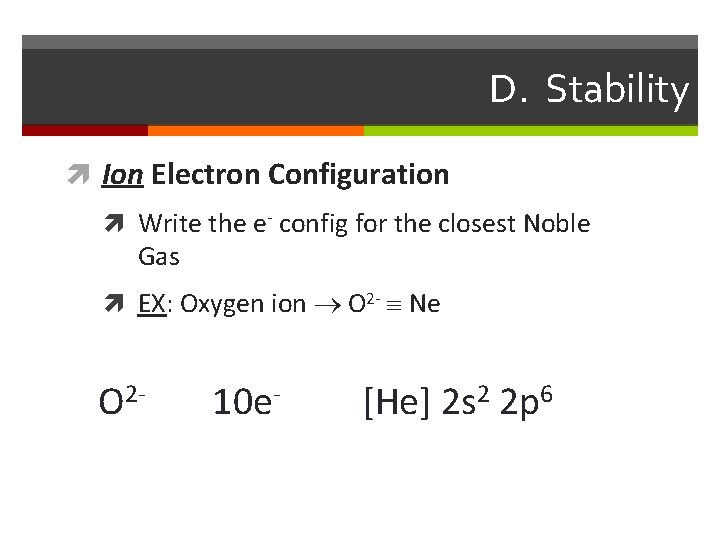
D. Stability Ion Electron Configuration Write the e- config for the closest Noble Gas EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6
- Slides: 65